Unexpected Vaginal Bleeding and COVID-19 Vaccination in Nonmenstruating Women
All Global Research articles can be read in 51 languages by activating the Translate Website button below the author’s name.
To receive Global Research’s Daily Newsletter (selected articles), click here.
Click the share button above to email/forward this article to your friends and colleagues. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
***
Abstract
The association between coronavirus disease 2019 (COVID-19) vaccination and vaginal bleeding among nonmenstruating women is not well studied. The Norwegian Institute of Public Health followed several cohorts throughout the pandemic and early performed a systematic data collection of self-reported unexpected vaginal bleeding in nonmenstruating women. Among 7725 postmenopausal women, 7148 perimenopausal women, and 7052 premenopausal women, 3.3, 14.1, and 13.1% experienced unexpected vaginal bleeding during a period of 8 to 9 months, respectively. In postmenopausal women, the risk of unexpected vaginal bleeding (i.e., postmenopausal bleeding) in the 4 weeks after COVID-19 vaccination was increased two- to threefold, compared to a prevaccination period. The corresponding risk of unexpected vaginal bleeding after vaccination was increased three- to fivefold in both nonmenstruating peri- and premenopausal women. In the premenopausal women, Spikevax was associated with at 32% increased risk as compared to Comirnaty. Our results must be confirmed in future studies.
Introduction
In the early fall of 2021, questions about bleeding disturbances and unexpected vaginal bleeding were included in questionnaires to several running Norwegian cohorts to explore free-text field comments from the participants shortly after introduction of the vaccine (18).
By use of questionnaire data from nearly 22,000 participants of the Norwegian Mother, Father, and Child Study (MoBa) (19) and the Senior cohort (20), we have investigated the association between COVID-19 vaccines and unexpected vaginal bleeding, i.e., (i) vaginal bleeding in postmenopausal women (e.g., PMB), (ii) unexpected vaginal bleeding in perimenopausal women, and (iii) breakthrough bleeding in nonmenstruating premenopausal women.
Results
The results are based on self-reported data from questionnaires issued in August and September 2021.
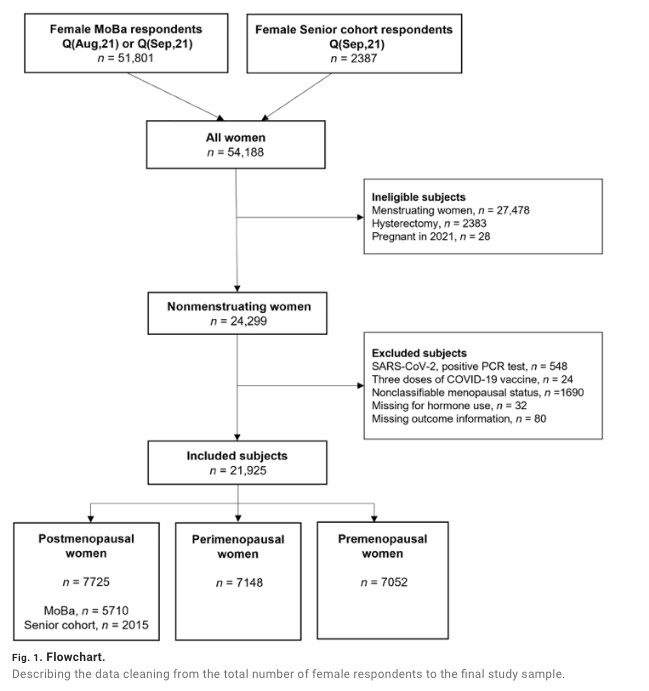
All female Senior cohort participants (ages 66 to 81 years) were considered nonmenstruating. Women who reported having had a hysterectomy were ineligible. After exclusion, the remaining eligible women (n = 2015) were allocated to the postmenopausal category (Fig. 1).
All female MoBa participants (ages 32 to 64 years) were asked “Do you still menstruate” (Yes/No/Do not know). Women who stated that they were still menstruating (“Yes”) were not eligible for inclusion. Women who reported having had a hysterectomy or were pregnant in 2021 were also ineligible. Women who denied (“No”) or were uncertain (“Do not know”) whether they were still menstruating were included and were all considered nonmenstruating.
The nonmenstruating MoBa participants were further categorized as post-, peri-, or premenopausal based on the response to three questions. They were defined as postmenopausal if they stated to have entered the menopausal transition, confirmed that their menstruations had stopped completely, and reported that their last menstruation occurred in 2019 or before (i.e., at least 1 year and 8 months prior) (n = 5710). Women were defined as perimenopausal if they stated to have entered the menopausal transition, confirmed that their menstruations had stopped completely, and reported that their last menstruation occurred in 2020 or 2021. Other combinations, including if they confirmed having entered the menopausal transition but denied or were uncertain whether their menstruations had stopped completely, also qualified for perimenopause (n = 7148). All nonmenstruating women who denied having entered the menopausal transition were defined as premenopausal (n = 7052). Age and reported hormone use was not applied in the categorization. See Materials and Methods for the complete description. A total of 21,925 participants from both cohorts were included (Fig. 1).
The median age of post-, peri-, and premenopausal women was 56, 52, and 45 years, respectively (Table 1). The vast majority received their first (98.0 to 98.4%) and second (91.5 to 95.0%) vaccine doses during the period covered by the questionnaire (1 January 2021 to the date of filling in the questionnaire). In post-, peri-, and premenopausal women, any hormone/contraception use was reported among 26.5, 57.2, and 85.5%, respectively. Among postmenopausal women, 13.7 and 7.4% reported using hormone replacement therapy (HRT) in MoBa and the Senior cohort, respectively (table S1). Most women in the premenopausal category reported having a hormonal intrauterine device (IUD) (74%). A medical history of any gynecological condition was reported among 14.5 to 19.3%. Further details of hormone use and gynecological conditions are shown in table S1.
Click here to expand for more data.
All nonmenstruating women were asked whether they had experienced unexpected vaginal bleeding in 2021 (i.e., the year the COVID-19 vaccination campaign was initiated) and whether this happened before or after COVID-19 vaccination. There were 252 (3.3%) postmenopausal women, 1008 (14.1%) perimenopausal women, and 924 (13.1%) premenopausal women who reported of unexpected vaginal bleeding during 2021. Of those who reported unexpected vaginal bleeding, 45, 51, and 55% of the post-, peri-, and premenopausal women, respectively, reported that the bleeding occurred within 4 weeks after the first and/or second vaccine dose. Perimenopausal women more often characterized the bleeding as heavy (27.9%) as compared to post- and premenopausal women (18.3 and 18.0%, respectively) (Table 2). In all three groups, bleeding after vaccination was more often characterized as heavy as compared to before vaccination. Perimenopausal women reported the longest bleeding duration, and in all groups, bleeding episodes were generally reported with slightly longer duration after vaccination compared to before vaccination. Similarly, the proportion of women who experienced only one bleeding episode was higher after vaccination in all three groups. The overall proportion who sought health care was higher among postmenopausal women compared to the peri- and premenopausal (30.6% versus 13.8% and 9.3%, respectively). Women more rarely sought health care when bleeding was reported to have occurred during the first 4 weeks after vaccination, as compared to before vaccination, in all three groups.
Click here to expand for more data.
Prevaccination rates of unexpected vaginal bleeding in post-, peri-, and premenopausal women were 4.0, 13.4, and 11.5 per 100 person years, respectively (Table 3). Compared to before vaccination, age-adjusted hazard ratios (aHRs) after the first and second dose in postmenopausal women were 3.0 [95% confidence interval (CI), 2.0 to 4.4] and 2.2 (95% CI, 1.4 to 3.5), respectively. In perimenopausal women, the corresponding aHRs were 4.2 (95% CI, 3.5 to 5.2) and 3.7 (95% CI, 2.9 to 4.7), and 4.7 (95% CI, 3.8 to 5.7) and 4.2 (95% CI, 3.3 to 5.2) in premenopausal women. In all groups, the rates observed more than 4 weeks after the first dose were consistently lower than the prevaccination rates. The risk estimates were virtually unchanged by additional adjustment (table S2). Direct comparison of the four postvaccination weeks after any dose of Spikevax against Comirnaty (reference), showed a 32% increased risk after Spikevax in premenopausal women [aHR, 1.32 (95% CI, 1.05 to 1.65)] (Table 4). Stratified analyses according to history of gynecological condition(s) consistently showed higher rates in women with any gynecological condition, whereas HRs were slightly higher for those without any such condition (table S3). In postmenopausal women, the proportions who reported unexpected vaginal bleeding declined rapidly according to the year of last menstruation both before and after vaccination (Table 5). In all groups of women, rates were higher in HRT users and women with hormonal IUD as compared to women not using hormones (Table 6). In postmenopausal women, the HRs were similar in nonhormone users and users of HRT; HRs were 2.9 (95% CI, 1.7 to 4.9) and 2.8 (95% CI, 1.5 to 5.2), respectively. In perimenopausal women, the HR was higher in nonhormone users [4.9 (95% CI, 3.6 to 6.6)] as compared to women using HRT [2.9 (95% CI, 1.7 to 5.0)] or hormonal IUD [3.8 (95% CI, 2.9 to 5.1)]. In premenopausal women, where hormonal IUD was the most common, the HRs were similar across categories of hormone use. When postmenopausal women were stratified according to early and late menopause (here, defined as ≤5 years and ≥6 years since last menstrual bleeding), the HR of PMB was slightly higher in nonhormone users in the early menopause [3.3 (95% CI, 1.7 to 6.4)] as compared to nonhormone users in late menopause [2.2 (95% CI, 0.9 to 5.6)] (Table 7).
Click here to expand for more data.
Click here to expand for more data.
Discussion
By use of data from two large population-based cohorts, we have observed an increased risk of unexpected vaginal bleeding after COVID-19 vaccination in nonmenstruating women across different stages of reproductive aging. Among post-, peri-, and premenopausal women, 3.3, 14.1, and 13.1% reported having one or several unexpected vaginal bleeding episodes during the last 8 to 9 months, of which approximately 50% were reported to have happened within 28 days of vaccination. In postmenopausal women, the risk of vaginal bleeding was increased two to threefold in the 4 weeks after vaccination, as compared to the prevaccination period. The association with vaccination was slightly stronger in peri- and premenopausal women where the risk was increased three to fivefold. In premenopausal women, the first 4 weeks after a dose of Spikevax was associated with a 32% increased risk as compared to Comirnaty.
Incidence rates of PMB in the population vary in previous publications, ranging from 0.2 to 1.5 per 100 person years (age dependent) based on hospital diagnoses (12, 21) to 13 per 100 person years in a 1-year daily diary study (22). Although not directly comparable, it is reassuring that our baseline estimate of PMB (i.e., 4.0 per 100 person years) lies between the estimates from these two approaches. Few studies have investigated the association between COVID-19 vaccination and unexpected vaginal bleeding in nonmenstruating women (11, 12, 17, 23), and PMB after COVID-19 vaccination has rarely been addressed (11, 12, 17, 23, 24).
Cross-sectional studies have reported higher frequencies of unexpected bleeding after COVID-19 vaccination compared to our study (17, 24). A small survey of pre- and postmenopausal women found that 11 and 38% of the postmenopausal women reported “menstrual symptoms” after the first and second dose, respectively (24). In a large sample recruited from social media, unexpected bleeding after vaccination was reported among 70% of women aged 18 to 45 years using long-acting reversible contraceptives and among 66% of postmenopausal women aged ≥55 years (17). In comparison, in the present study of previously enrolled cohort participants, the proportions of women with unexpected vaginal bleeding within 4 weeks of vaccination were 7.4% for premenopausal women and 1.5% for postmenopausal women. As acknowledged by Lee et al. (17), having experienced any of these outcomes probably increased the likelihood of participation in their study. Of note, a small survey in Japanese health care workers reported that among 103 postmenopausal women, none had reported irregular bleeding after vaccination (25).
In agreement with our findings, two large studies from the United States (11) and Sweden (12) using health record systems found positive associations between COVID-19 vaccination and PMB. The risk of a PMB diagnosis was increased by 21 and 14% respectively, when compared to prevaccination periods. In our cohort, only 31% of women who reported a PMB sought medical care, and the proportion was even lower if the bleeding occurred after vaccination. Thus, lower risk estimates are expected from a diagnosis-based approach. Furthermore, the defined risk windows were longer than the 28 days in our study (i.e., 82 to 112 days) (11, 12).
Two of the abovementioned studies saw no clear difference in bleeding reports according to vaccine type (12, 17). However, the Spikevax vaccine used in primary vaccination (first and second doses) contains a higher dose of mRNA (100 μg) as compared to the Comirnaty vaccine (30 μg) and has been associated with higher rates of adverse events, in particular at younger age (26–29). In line with this, we observed a higher risk of vaginal bleeding after Spikevax as compared to Comirnaty in premenopausal women. Also, a study analyzing the free-text fields of unsolicited reactions after COVID-19 vaccination in the CDC v-safe surveillance system found that a larger proportion of respondents with PMB had received the Spikevax vaccine than expected if vaccine type were independent (23).
After the menopause, the endometrium normally undergoes a gradual atrophy, starting with an inactive phase in which neither proliferation nor secretion is present and ending in a thin layer, often with cystic cavities (30). HRT, most commonly a combination of estrogen and progestogen, may interfere with the physiological atrophy (30, 31), and vaginal bleeding is a common side effect (32). Ljung et al. (12) observed a slightly stronger association between vaccination and PMB after the third dose in a subsample analysis of nonhormone users as compared to the analysis on the complete sample. In our study, the strength of the association between vaccination and PMB was similar in HRT users and nonhormone users. However, we observed a slightly stronger association between vaccination and vaginal bleeding in nonhormone users who more recently entered menopause, but the CIs were wide. In HRT users, the strength of the association was similar irrespective of time elapsed since the last menstrual bleeding. Given that HRT stimulates the endometrium and may delay the endometrial atrophy, this finding seems reasonable. However, care should be taken in the interpretation due to small groups and the nonrandom distribution of hormone use (i.e., the hormone use per se cannot be distinguished from the indication).
An irregular bleeding pattern is the clinical hallmark of perimenopause. According to the Stages of Reproductive Aging Workshop (STRAW) criteria for staging reproductive aging (33), perimenopause begins at stage −2 (early menopausal transition), characterized by increased variability in menstrual cycle length, and ends 12 months after the final menstrual period. It is therefore reassuring that perimenopausal women had the highest prevaccination bleeding rates in the study. However, despite the high baseline rates (reference), the association with vaccination was not weaker in this group. In perimenopause, the strongest association between vaccination and bleeding was observed among nonhormone users, but the CIs were wide.
Most nonmenstruating premenopausal women in our study had a hormonal IUD (74%). Thus, the overall estimates for the premenopausal group reflect women with such device. Common endometrial changes in these women are glandular atrophy and stromal decidualization, in addition to a foreign body reaction characterized by an increase in inflammatory cells. Breakthrough bleeding is common, in particular during the initial period after insertion (34). However, despite the physiological changes, we did not detect clear differences in the relative risk of bleeding across hormone use in premenopausal women.
In all three groups, the association between vaccination and bleeding tended to be slightly stronger in women without gynecological conditions as compared to women with such history. Yet, as the rates were generally higher among women with any gynecological condition, the absolute excess risk posed by vaccination was greater among women with these conditions.
Our findings indicate that the COVID-19 vaccines, or the host response to them, can lead to vaginal bleeding in a wide range of women. Unexpected vaginal bleeding in post-, peri-, and premenopausal women generally have different underlying causes. However, our findings of an increased risk across the reproductive stages raise the possibility that the mechanisms linking COVID-19 vaccination to unexpected vaginal bleeding may be similar across the stages. Although our data are not fit to explore biological mechanisms, the increased risk after vaccination across different stages of reproductive aging (i.e., in post-, peri-, and premenopausal women) and exogenous hormone use may suggest that the mechanism is not through disruptions of the hypothalamic-pituitary-ovarian axis. Increased risk after both Comirnaty and Spikevax suggest a mechanism related to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein and not to other vaccine components. This is also supported by a higher risk observed after Spikevax in premenopausal women. An increased risk of PMB diagnosis after SARS-CoV-2 infection has also been described (12), further supporting a role of the viral agent. Pathways related to local changes in the endometrium, possibly resulting from a spike related immune response or related to the endometrial expression of angiotensin-converting enzyme 2 (ACE2) receptors (i.e., the receptor for the SARS-CoV-2 spike protein) may be involved (17, 35–37). However, a general bleeding tendency after vaccination cannot be ruled out.
Major strengths of this study are the large sample, high response rates, and the reduced risk of selection bias as participants were already enrolled at the time of vaccination. Being self-reported, outcome frequencies are more complete than if limited to medical diagnoses. Although it can be argued that participants who had experienced any kind of adverse event would be more motivated to return their questionnaire, the questionnaires covered a wide spectrum of other health- and pandemic-related topics, not specifically targeting adverse events after vaccination. Also, the response rates were similar in preceding and subsequent questionnaires, and the sensitivity analysis for the subpopulation complete for all covariates showed almost identical results. The study collected data on the time elapsed from vaccination to the bleeding event. Unlike diagnosis-based studies, which must investigate a longer time-period due to diagnosis delay (11, 12), we could calculate rates within a biologically plausible time interval of 4 weeks postvaccination. The study was conducted early in the pandemic, before the Omicron surge, and therefore, only to a minimal degree influenced by unrecognized SARS-CoV-2 infection. We also had information on important characteristics such as hormone treatment, hysterectomy, and gynecological conditions, as well as body mass index (BMI) and educational level for a large subsample (80%), allowing for relevant categorizations and sensitivity analysis with correction for potential confounders. We had information on menopausal status and did not have to use an arbitrary age limit with inevitable misclassification (38).
Our study has some important limitations. Outcomes were retrospectively collected and vulnerable to recall bias. More complete reporting postvaccination compared to the prevaccination period may have existed, and differential misclassification in the sense of a higher proportion of true cases classified as noncases in the reference period could have influenced the results. Participants were asked to state if their change in bleeding occurred after vaccination, and further time precision was defined from time elapsed from the vaccination date. The risk more than 4 weeks after the first dose was lower than that of the reference period. This is most likely the result of a timing-related misclassification. This tendency disappeared after the second dose, which may suggest that more recent events were more accurately allocated. However, as a COVID-19 vaccine potentially could have triggered an endometrial breakdown/bleeding (which otherwise would have happened within weeks/months), the lower risk could also be a true observation. Media attention could have introduced further bias in the reporting. However, in contrast to menstrual changes, which in a sense are subjective and can be influenced by awareness, we believe that unexpected bleeding in nonmenstruating women represents a more robust event that most women are likely to remember and be able to report quite accurately. Presumably, this is especially true for postmenopausal women. It is also possible that the media attention was helpful, as women would be more likely to remember when their own bleeding occurred, in relation to their vaccines (i.e., before or after). Supporting our hypothesis of reliable reporting, our data show clear expected trends of bleeding rates across menopausal status and year since last menstruation (22). Furthermore, the observed increased risk after Spikevax compared to Comirnaty, which is not unreasonable due to the higher mRNA dose, is unlikely to have been influenced by differential recall bias or awareness. Last, some misclassification of the reproductive stages is necessarily present. The classification was based on self-reported information and partly required that respondents were familiar with the term “menopausal transition.” While nearly 85% of the women assigned to the premenopausal category reported use of hormonal contraception, thus providing a reasonable explanation as to why they did not menstruate, we know less about the remaining 15%. Probably, this subgroup contains women misclassified to the category as well as women with amenorrhea due to other cause (hysterectomized and pregnant women were not eligible). The menopausal transition may be difficult for women to clinically recognize and the perimenopausal category was therefore also broadly defined in our study. Furthermore, because information about the participants’ last menstrual bleeding was available in years only, and a cutoff of 2019 was used to ensure true postmenopausal status (i.e., 12 months or more since their last menstrual bleeding), some women in the early postmenopause have been assigned perimenopausal status. Women in the early menopausal transition have not been fully addressed in this study. As this period is defined by increased variability in menstrual cycle length, and not amenorrhea, we expect that women in this stage, who were not amenorrheic due to exogenous hormones, reported to still be menstruating and therefore not eligible.
Some aspects might influence the generalizability of our results. First, the cohort participants are not completely representative of the general population. Participants have a higher educational level (20, 39) and are probably more health conscious as compared to the general Norwegian population. Reassuringly, investigation of self-selection in MoBa has suggested that while prevalence estimates of exposures and outcomes may be biased, estimates of exposure-outcome associations are not (40). We do not expect that the selection into the cohorts introduces substantial bias to our estimates in this study. Secondly, MoBa, representing 91% of our study sample, is a pregnancy-based cohort, and thus, most women in our study have been pregnant at least once. However, although pregnancies cause some structural and functional changes to the uterus (41, 42), we do not suspect that the association between COVID-19 vaccination and vaginal bleeding would be markedly different in nulliparous women. Of note, menstrual disturbances after vaccination have been reported in nulliparous women from the age of 12 years (18, 43).
PMB represents an important medical event that cannot be explained by circumstantial factors such as pandemic-related stress. Thus, the finding of increased risk of PMB is a strong advocate for a true biological effect of vaccination on female bleeding patterns overall. Since PMB also has clinical implications in the sense of elaborated diagnostics and severe patient concerns, clarification of an association is imperative.
We believe that this study, which focuses on major groups of women rarely included in related studies, offers an important contribution to the current body of evidence within this field. In our sample of health-conscious women, only 31, 14, and 9% of the post-, peri-, and premenopausal women with reported bleeding also reported that they sought medical care, respectively. This health-seeking behavior also differed by vaccination status. This illustrates the role of self-reported data in the investigation of certain end points. While bias may partly explain the association in this retrospective analysis, we do not believe that it accounts for all the increased risk we observed. Together with current knowledge, it seems probable that both pre- and postmenopausal women are at increased risk of unexpected vaginal bleeding after COVID-19 vaccination. Our findings must be confirmed by well-designed prospective studies and such events should be addressed in clinical trials of future vaccines.
Materials and Methods
We used data from two cohorts administered by the Norwegian Institute of Public Health, namely, the MoBa and the Senior cohort. The MoBa is an ongoing, nationwide population-based pregnancy cohort with recruitment from 1999 to 2008 (19). Mothers consented to participate in 41% of the pregnancies. Since March 2020, adult participants have been invited to answer electronic questionnaires with questions related to the SARS-CoV-2 pandemic every 14 days. Questions about bleeding disturbances and unexpected vaginal bleeding were included in two consecutive questionnaires, distributed to 103,904 and 103,791 participants on 20 August and 1 September 2021 [Q(Aug,21) and Q(Sep,21)]. The response rates were high (71 and 72%, respectively), and most of the respondents returned the questionnaire on the date of distribution (61 and 62%, respectively).
The Senior cohort was established in December 2020 to cover older age groups during the pandemic. About 13,000 randomly selected citizens of Oslo aged 65 to 80 years were invited, and 36% consented to participation. To date, eight electronic questionnaires have been distributed. Gynecological history and unexpected vaginal bleeding were covered in the questionnaire distributed to 4814 subjects on 23 September 2021 [Q(Sep,21)]. The response rate was 95 and 54% returned the questionnaire on the distribution date.
In MoBa, we used information from Q(Sep,21) (n = 46,356), and if not available, we added responses from Q(Aug,21) (n = 5445) (Fig. 1). The number of female respondents to Q(Sep,21) in the Senior cohort was 2387. All Senior cohort participants were considered nonmenstruating. In MoBa, all women were asked “Do you still menstruate?” (Yes/No/Do not know). Women who answered “Yes” were ineligible for inclusion in the present study (n = 27,478). In both cohorts, women with reported hysterectomy (n = 2383) or pregnancy in 2021 (n = 28) were also ineligible. The eligible study population of nonmenstruating women consisted of 24,299 subjects.
The study was approved by The Regional Committee for Medical and Health Research Ethics, Southeast Norway. Written informed consent was obtained from all participants.
Exposure
Vaccination dates and the type of vaccine against COVID-19 was obtained through linkage with the Norwegian Immunization Registry by use of each participant’s unique national identity number. Notification to the registry is mandatory and performed by the personnel providing the vaccines at the time of vaccination. A time-dependent exposure variable was created by use of vaccination dates [i.e., unvaccinated/prevaccination; first 4 weeks after dose 1; more than 4 weeks after dose 1 (but before dose 2); first 4 weeks after dose 2; more than 4 weeks after dose 2].
Outcome
Covariates and categorization
Perimenopausal women includes women who confirmed having entered the menopausal transition (“Yes”) and confirmed that their menstruations had stopped completely (“Yes”) but provided a year of last menstruation of 2020 or 2021. Women were also allocated to this category if they (i) confirmed having entered the menopausal transition (“Yes”) and denied that their menstruations had stopped completely (“No”), (ii) confirmed having entered the menopausal transition (“Yes”) and were uncertain whether their menstruations had stopped completely (“Do not know”), (iii) were uncertain whether they had entered the menopausal transition (“Do not know”) and were uncertain whether their menstruations had stopped completely (Do not know), and (iv) were uncertain whether they had entered the menopausal transition (“Do not know”) and denied that their menstruations had stopped completely (“No”).
Premenopausal women includes women who denied having entered the menopausal transition (“No”), irrespective of their response to whether their menstruations had stopped completely (i.e., “Yes,” “No,” or “Do not know”).
Study sample
Design and statistical analyses
Since women were asked about bleeding events during 2021, all the women were followed from 1 January 2021. End of follow-up was the fill in date of the questionnaire or the estimated date of bleeding, whichever occurred first. We used Cox regression to estimate the association between vaccination and risk of unexpected bleeding. The model was adjusted for age as a continuous variable. In addition, a multivariate model (adjusted for age, hormone use, BMI category, educational level, and any gynecological condition), and crude and age-adjusted analyses were performed for a subset of participants with complete information on all covariates. In a separate analysis, the first 4 weeks after a dose of Spikevax was compared to the first 4 weeks after Comirnaty. The main analyses were stratified according to any gynecological conditions and certain categories of hormone use. Postmenopausal women were also stratified according to the number of years since last menstrual bleeding. Because of power limitations, in the stratified analyses, the first and second doses were combined. Statistical analyses were performed in STATA version 17.0.
*
Note to readers: Please click the share button above. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
Click here to see the references.
Featured image is from NaturalNews.com
 The Worldwide Corona Crisis, Global Coup d’Etat Against Humanity
The Worldwide Corona Crisis, Global Coup d’Etat Against Humanity
by Michel Chossudovsky
Michel Chossudovsky reviews in detail how this insidious project “destroys people’s lives”. He provides a comprehensive analysis of everything you need to know about the “pandemic” — from the medical dimensions to the economic and social repercussions, political underpinnings, and mental and psychological impacts.
“My objective as an author is to inform people worldwide and refute the official narrative which has been used as a justification to destabilize the economic and social fabric of entire countries, followed by the imposition of the “deadly” COVID-19 “vaccine”. This crisis affects humanity in its entirety: almost 8 billion people. We stand in solidarity with our fellow human beings and our children worldwide. Truth is a powerful instrument.”
Reviews
This is an in-depth resource of great interest if it is the wider perspective you are motivated to understand a little better, the author is very knowledgeable about geopolitics and this comes out in the way Covid is contextualized. —Dr. Mike Yeadon
In this war against humanity in which we find ourselves, in this singular, irregular and massive assault against liberty and the goodness of people, Chossudovsky’s book is a rock upon which to sustain our fight. –Dr. Emanuel Garcia
In fifteen concise science-based chapters, Michel traces the false covid pandemic, explaining how a PCR test, producing up to 97% proven false positives, combined with a relentless 24/7 fear campaign, was able to create a worldwide panic-laden “plandemic”; that this plandemic would never have been possible without the infamous DNA-modifying Polymerase Chain Reaction test – which to this day is being pushed on a majority of innocent people who have no clue. His conclusions are evidenced by renown scientists. —Peter Koenig
Professor Chossudovsky exposes the truth that “there is no causal relationship between the virus and economic variables.” In other words, it was not COVID-19 but, rather, the deliberate implementation of the illogical, scientifically baseless lockdowns that caused the shutdown of the global economy. –David Skripac
A reading of Chossudovsky’s book provides a comprehensive lesson in how there is a global coup d’état under way called “The Great Reset” that if not resisted and defeated by freedom loving people everywhere will result in a dystopian future not yet imagined. Pass on this free gift from Professor Chossudovsky before it’s too late. You will not find so much valuable information and analysis in one place. –Edward Curtin
ISBN: 978-0-9879389-3-0, Year: 2022, PDF Ebook, Pages: 164, 15 Chapters
Price: $11.50 FREE COPY! Click here (docsend) and download.
We encourage you to support the eBook project by making a donation through Global Research’s DonorBox “Worldwide Corona Crisis” Campaign Page.