The Pfizer mRNA Vaccine: Pharmacokinetics and Toxicity
All Global Research articles can be read in 51 languages by activating the “Translate Website” drop down menu on the top banner of our home page (Desktop version).
Visit and follow us on Instagram at @crg_globalresearch.
***
Abstract
We summarize the findings of an animal study which Pfizer submitted to the Japanese health authorities in 2020, and which pertained to the distribution and elimination of a model mRNA vaccine. We show that this study clearly presaged grave risks of blood clotting and other adverse effects. The failure to monitor and assess these risks in the subsequent clinical trials, and the grossly negligent review process in conjunction with the emergency use authorizations, have predictably resulted in an unprecedented medical disaster.
1. Introduction and background
As with any drug, a key consideration for the toxicity of the COVID mRNA vaccines is where exactly in the body they end up, and for how long they will stay there. Such questions, which are the subject of pharmacokinetics, are usually thoroughly investigated and during drug development. Initial studies on pharmacokinetics and also on toxicity are carried out in animals. If the outcome is favourable, similar experiments will be performed on a small number of human volunteers. Only after such preliminary studies have been successfully concluded will proper clinical trials be approved, which will then determine whether the drug or vaccine in question has the desired clinical efficacy.
Because of the officially sanctioned haste and systematic gross negligence in the development and approval of the COVID-19 vaccines, our knowledge of their pharmacokinetics is sketchy. The only somewhat detailed animal study that has reached the public pertains to the Pfizer vaccine [1, 2]. These data were publicized after Pfizer had filed them with the Health authorities in Japan when applying for emergency use authorization of its vaccine in that country.1 These data pertained in particular to the distribution of the vaccine within the body after injection and to its elimination from the body. Even though far from being comprehensive or even adequate, this document has rather far-reaching implications: it shows that Pfizer— as well as the authorities that were apprised of these data— must have recognized the grave risks of adverse events after vaccination even before the onset of clinical trials. Nevertheless, Pfizer’s own clinical trials failed to monitor any of the clinical risks that were clearly evident from these data, and the regulatory authorities failed to enforce proper standards of oversight. This dual failure has caused the most grievous harm to the public.
Before we discuss this study and its implications in detail, we will briefly review how the Pfizer mRNA vaccine works. These explanations also apply to the Moderna mRNA vaccine, whereas the AstraZeneca and the Johnson & Johnson vaccines differ in some aspects.
1.1 How the mRNA COVID vaccines work
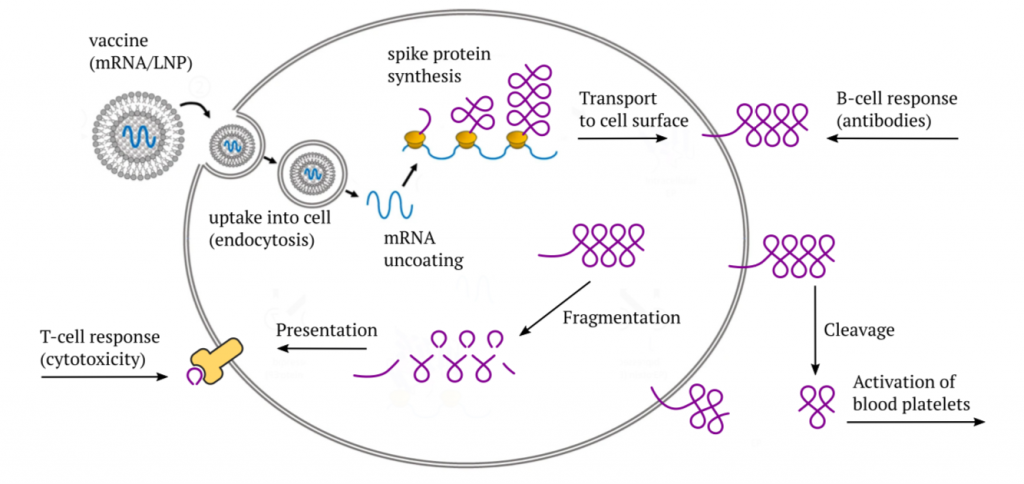
The Pfizer and Moderna mRNA vaccines consist of a synthetic messenger RNA (mRNA) that encodes the SARS-CoV-2 “spike protein,” which normally is found on the surface of the coronavirus particles. This mRNA is coated with a mixture of synthetic lipids (fat-like molecules) that protect it during transport within the body, and which also facilitate its uptake into the target cells through endocytosis.
After the vaccine has entered a cell, it initially finds itself enclosed by a mem- brane vesicle—a little bubble that was pinched off from the cell membrane. The subsequent accumulation of acid inside this bubble causes the lipids to be stripped off, and the mRNA to be released into the cytosol (the intracellular fluid); this release step is facilitated by the cationic lipid ALC-0315 (see later). The mRNA then binds to ribosomes—the cell’s little protein factories—and induces the synthesis of the actual spike protein molecules. Most of the spike protein molecules will then be transported to the cell surface.
Once it appears there, it will be recognized by B-lymphocytes (B-cells), which will then start making antibodies to it.2 Furthermore, some part of the spike protein can also be cleaved off by proteases on the cell surface and released from the cell. If this happens within the circulation, the released fragment—referred to as S1—can bind to blood platelets (thrombocytes) and activate them. In this manner, the spike protein directly promotes blood clotting.
As with any protein that is synthesized within the cell, a small number of mole- cules will undergo fragmentation, and the fragments will be presented on the cell surface in association with specific (HLA-) carrier proteins. The purpose of this mechanism is immune surveillance—as soon as fragments show up of some protein which the immune system does not recognize as “self,” an immune response will be mounted against that protein and against the cells that produce it. This response is mediated by cytotoxic T-lymphocytes (CTLs, T-killer cells).
In mounting its cytotoxic response, the immune system will not distinguish between a true virus infection and the expression of an mRNA vaccine—as long as the spike protein fragments appear on the cell, the killer cells will be on the march. If the vaccine is expressed in the cells that line the blood vessels—the endothelial cells—the vascular lesion caused by the immune attack will again set off blood clotting. Thus, we have at least two distinct paths toward blood clotting after vaccination.
1.2 The lipid-coated mRNA vaccines acquire an apolipoprotein “corona”
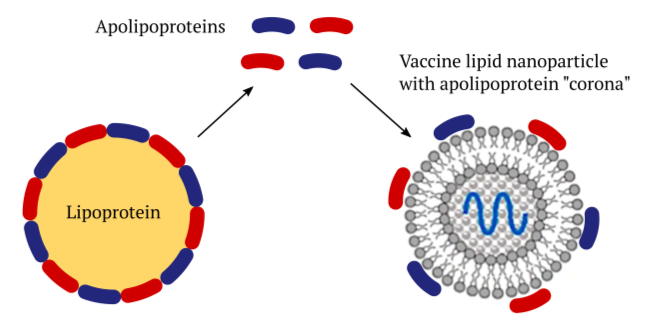
Lipoprotein particles occur naturally in the bloodstream and within the tissues of our body. They consist of a core of lipids that is surrounded with a shell of proteins called apolipoproteins. Their purpose is to transport lipids such as cholesterol and triacylglycerol (regular fat) between organs. For example, a specific type of lipoprotein called chylomicrons transports dietary fats after they have been taken up in the small intestine. Other lipoproteins called VLDL and LDL distribute fats that have been synthesized in the liver to other organs and tissues.
The various apolipoproteins that encase the lipoproteins stabilize the particles, and they also serve as “address tags” that bind to receptor molecules on cell surfaces. This interaction will trigger the uptake of the lipoproteins into those cells. Artificial lipid nanoparticles (LNPs) like those used in the COVID mRNA vaccines can acquire a shell—a “corona”—of the body’s own apolipoprotein molecules [3]. This enables these vaccines to be taken up into the cells of our body, too.
The liver has a central place in lipid and lipoprotein metabolic turnover. Accordingly, liver cells are rich in specific surface receptor molecules which mediate lipoprotein uptake, suggesting that they will efficiently take up LNPs decorated with apolipoproteins also. This is indeed the case. However, other organs have high rates of lipoprotein uptake, too, and they must therefore be expected to accumulate the apolipoprotein-decorated vaccine LNPs as well.
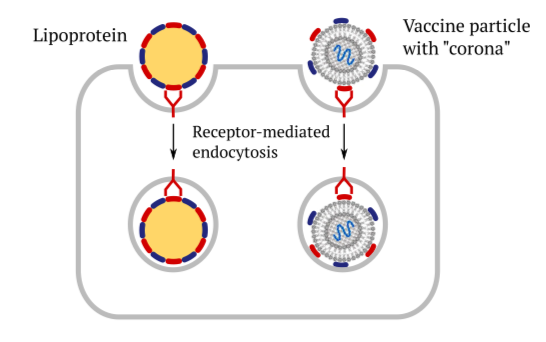
1.3 Receptor-mediated cellular uptake of lipoproteins and of vaccines
This slide illustrates the role of cellular receptors and the apolipoproteins in facili- tating the uptake of vaccines into cells through endocytosis. They bind to the same cellular receptors as the regular lipoprotein particles do, and they subsequently get taken up in the same manner. The subsequent events—release of the mRNA and protein synthesis—have already been discussed above.
1.4 Transcytosis of lipoproteins from the bloodstream into the tissues
All substrate exchange between the tissues and the bloodstream occurs in the capil- laries. In these finest of all blood vessels, the blood is separated from the extracel- lular matrix of the tissues by only one cellular layer—namely, the endothelial cells. The capillary wall permits free passage only to small molecules such as for example blood sugar (glucose) or amino acids. The lipoproteins, which are far larger, must be transported across the capillary wall by transcytosis. In this two-stage process, endocytosis on one side of the cell is followed by exocytosis, that is, by release of the particles, which occurs on the other side.
While this figure shows transcytosis from the bloodstream to the tissue, the process actually works in both directions. In this manner, cells in the tissues can avail themselves of cholesterol carried by circulating LDL, but they can also return surplus cholesterol through the bloodstream to the liver via other lipoproteins (HDL).
Transcytosis will also apply to the “corona”-decorated vaccine LNPs and enable them to reach the tissues in various organs. Reverse transcytosis of vaccine might contribute to its uptake from the muscle tissue into the circulation after injection (see below).
To Read the complete article click here