COVID-19 Vaccine and Myocarditis: New October 2023 Papers Raise Serious Concerns! The Good, the Bad and the Ugly Regarding Novavax “2023-2024 Formulation”

All Global Research articles can be read in 51 languages by activating the Translate Website button below the author’s name.
To receive Global Research’s Daily Newsletter (selected articles), click here.
Click the share button above to email/forward this article to your friends and colleagues. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
***
Papers Reviewed:
-
Oct. 26, 2023 Wilkinson et al – A Brighton Collaboration standardized template with key considerations for a benefit/risk assessment for the Novavax COVID-19 Vaccine (NVX-CoV2373), a recombinant spike protein vaccine with Matrix-M adjuvant to prevent disease caused by SARS-CoV-2 viruses
- Oct. 18, 2023 – COVID-19 Update: New Novavax Vaccine Formulation for 2023-2024
- June 2023 Smith et al – Safety of the NVX-CoV2373 COVID-19 vaccine in randomized placebo-controlled clinical trials
- Feb. 2023 Saint-Gerons et al – Myopericarditis Associated with the Novavax COVID-19 Vaccine (NVX-CoV2373): A Retrospective Analysis of Individual Case Safety Reports from VigiBase
- Dec. 2022 Ahmad et al – Myopericarditis following both BNT162b2 and NVX-CoV2373
-
June 7 2022 – U.S. Food and Drug Administration. Novavax COVID-19 vaccine (NVX-CoV2373) VRBPAC briefing document. Updated 2022
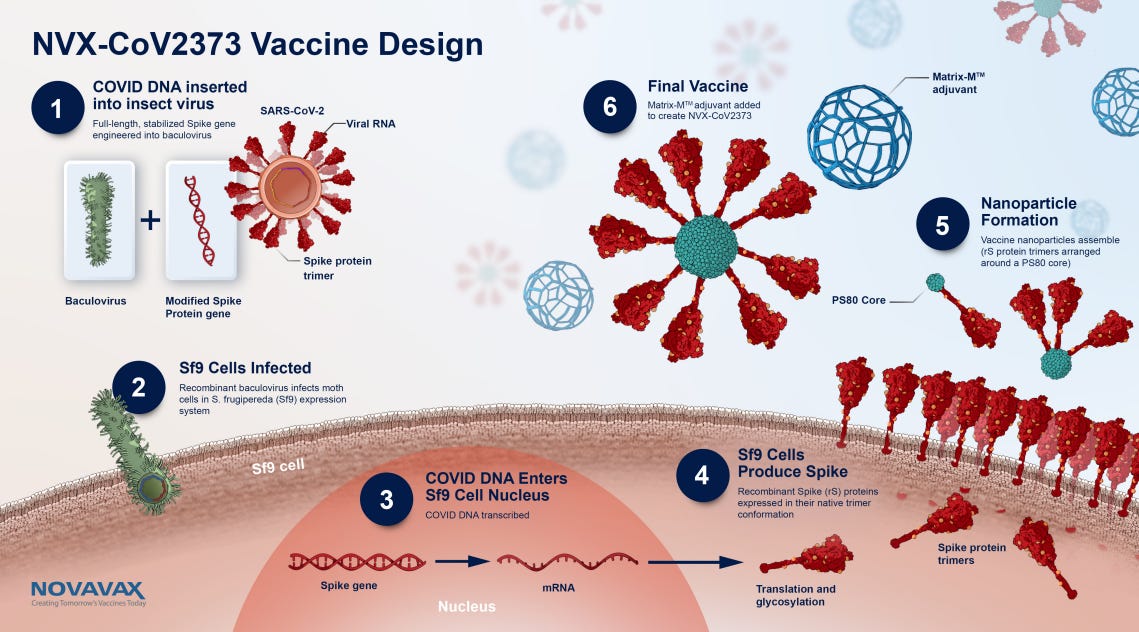
Oct. 26, 2023 Wilkinson et al – A Brighton Collaboration standardized template with key considerations for a benefit/risk assessment for the Novavax COVID-19 Vaccine (NVX-CoV2373), a recombinant spike protein vaccine with Matrix-M adjuvant to prevent disease caused by SARS-CoV-2 viruses.
Feb. 2023 Saint-Gerons et al – Myopericarditis Associated with the Novavax COVID-19 Vaccine (NVX-CoV2373): A Retrospective Analysis of Individual Case Safety Reports from VigiBase.
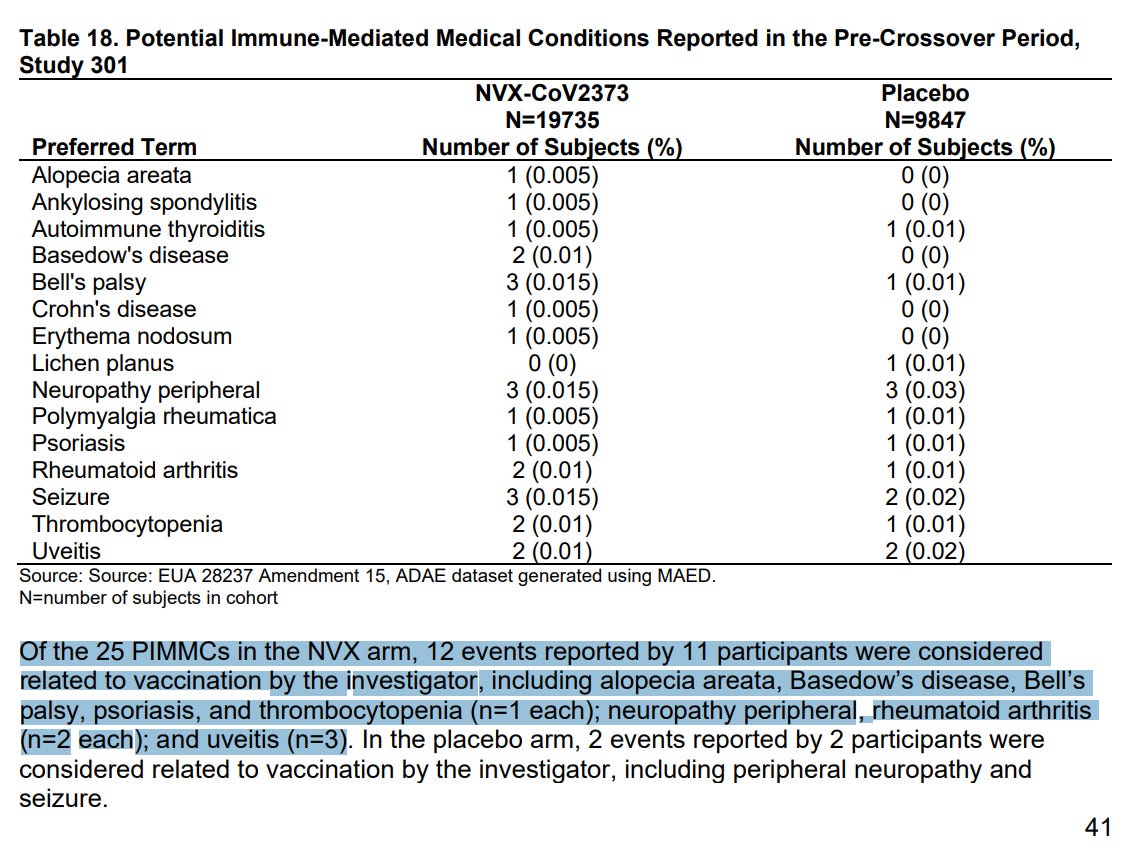
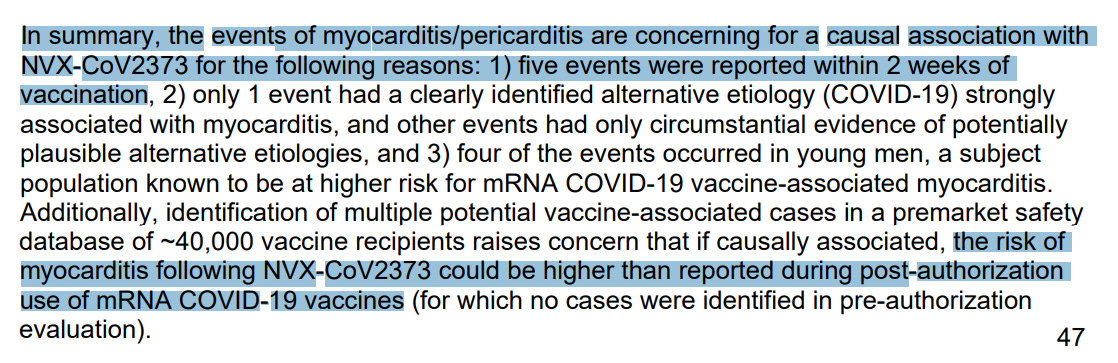
- 61 safety reports for myocarditis and pericarditis reported to regulators, 24 serious, 19 required hospitalization, 3 were life threatening, 1 disabling, none were fatal
- median age 36 years old, 62%, 38% women
- 70% had chest pain
- median period to onset 3 days after jab
- only 13% recovered
- “disproportionality signal was found for NVX-CoV2373 (Novavax) vaccine in line with the mRNA vaccines, and the Pfizer-BioNTech vaccine in particular”
- AstraZeneca had elevated risk of myocarditis but only 1/10th that of Pfizer or Novavax.
- “More research would be needed to understand the role of nanoparticles in the potential risk of vaccine-induced myocarditis.”
- Interpretation: Novavax has myocarditis risk comparable to Pfizer mRNA


 The Worldwide Corona Crisis, Global Coup d’Etat Against Humanity
The Worldwide Corona Crisis, Global Coup d’Etat Against Humanity