Low RSV Vaccine Acceptance Among Pregnant Women
Wary of Fever and Pregnancy Loss, Discerning Mothers Declining the Novel Shot

All Global Research articles can be read in 51 languages by activating the Translate Website button below the author’s name (only available in desktop version).
To receive Global Research’s Daily Newsletter (selected articles), click here.
Click the share button above to email/forward this article to your friends and colleagues. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
New Year Donation Drive: Global Research Is Committed to the “Unspoken Truth”
***
Vaccination during the third trimester of pregnancy is unprecedented and risky, since a vaccine induced fever could precipitate stillbirth or premature delivery of the baby. The CDC and the Bio-Pharmaceutical Complex has told young mothers they should take the risk for theoretical protect of the newborn.
As of August 30, 2023, the CDC recommends: “Vaccination for pregnant people, 1 dose of maternal RSV vaccine during weeks 32 through 36 of pregnancy, administered immediately before or during RSV season. Abrysvo is the only RSV vaccine recommended during pregnancy.” Now the CDC is reporting that only Asian women in the US have topped 10% on the respiratory syncytial virus RSV vaccination rate while African American mothers remain the most conservative with under 5% rates of acceptance. For any mass vaccination campaign, these data would indicate a program failure. The mothers and families have been burned by genetic COVID-19 vaccines and unprecedented rates of injury, disability, and death. There is little appetite for a new vaccine during pregnancy among obstetricians, midwives, and expecting mothers.
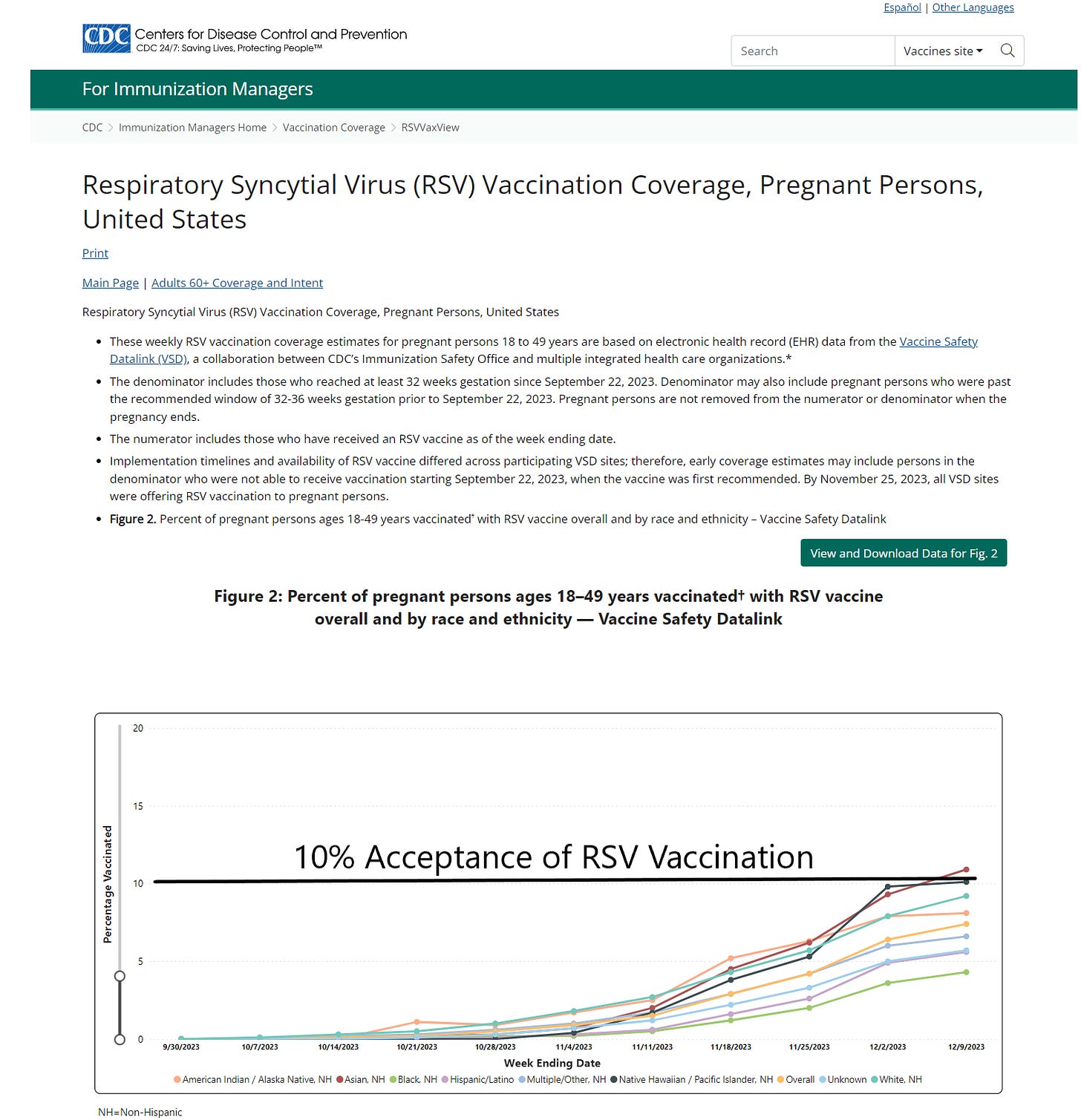
CDC.Gov accessed January 3, 2023
These data on the lagging maternal RSV immunization campaign indicate that “vaccine mania” may be cooling in the United States. As a consulting internist and cardiologist, I do not recommend the new RSV vaccine for pregnant women. There are insufficient data on short and longer term safety. Theoretical protection of infants for an easily treatable illness is simply not compelling enough to risk the pregnancy altogether.
*
Note to readers: Please click the share button above. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
Featured image is from Mercola

