Study of Long-term Effect of Fluoride Toothpaste on Human Teeth
All Global Research articles can be read in 51 languages by activating the Translate Website button below the author’s name.
To receive Global Research’s Daily Newsletter (selected articles), click here.
Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
***
Abstract
To study the long-term effects of Fluoride toothpaste on human teeth, a two-month experiment has been carried out to simulate 47 years of exposure on a human tooth to Fluoride toothpaste under normal daily usage. Micro-cracks have shown up on both Enamel and Cementum surfaces, which is a sign of tooth degradation. While the Cementum surface has a much higher density of micro-crack networks. Cementum tends to get harmed by Fluoride more often than Enamel. This study does not include other factors such as chewing and the type of food residue stuck between teeth, which could potentially affect the tooth’s health even further.
Introduction
Our teeth are a crucial part of our body. We need them to be able to masticate our food into smaller pieces for easier digestion. When we masticate, sometimes small pieces of food can get stuck on our teeth. In order to clean our teeth, we will commonly brush our teeth around 2-3 times a day with toothpaste. Most commercial toothpastes (such as Crest® or Colgate®) we see in our supermarkets contain an ingredient known as Fluoride, with a typical percentage of 0.25%, while some brands contain up to 0.75% Fluoride. Prescription Dental toothpastes can contain 5 times more Fluoride than commercially available.
Although most Americans have positive associations with fluoride and envision tooth protection along with strong bones, fluoride is actually the by-product of industry and its toxicity was recognized at the beginning of the Industrial Revolution during the 1850’s, when iron and copper factories discharged it into the air and poisoned plants, animals, and people. The fluoride added to drinking water and toothpaste is a crude industrial waste product of the aluminum and fertilizer industries, and a substance toxic enough to be used as rat poison. [1]
Fluoride has been used in the US to treat drinking water and added to toothpaste, all of which started from a carefully planned marketing program that began before the Grand Rapids, Michigan, which became the first community to officially fluoridate its drinking water in 1945 [2]. The big hope for fluoride was its ability to immunize children’s developing teeth against cavities. Rates of dental caries were supposed to plummet in areas where water was treated, yet decades of experience and worldwide research have contradicted this expectation numerous times. Let alone overdosed fluoridation has been linked to Skeletal Fluorosis [3], Dental Fluorosis [4], Bone Fractures [5] [6], Fluoride Poisoning [7], Cancer [8] [9].
Since we are extremely concerned about the usage of Fluoride in the toothpaste commonly seen, an experiment has been planned to study the effect of Fluoride on human teeth under long term condition.
Materials & Methods
To figure out if fluoride contained in fluoride toothpaste is harmful to our teeth or not, an experiment of human tooth’s long-time exposure to fluoride toothpaste has been designed and carried out to monitor the change of tooth surface.
To simulate years’ usage of fluoride toothpaste on a human tooth, a real human tooth has been coated with prescription toothpaste for 2 months, which is equivalent to 47 years of exposure if 5 minutes of brushing with toothpaste per day is assumed as normal daily usage. The prescription toothpaste used here is Rising® Denta 5000 Plus 1.1% Sodium Fluoride Prescription Dental Cream (5000 ppm Fluoride Plus Mild Cleaning System). The human tooth had been cleaned with distilled water and Acetone, and then recoated with this prescription toothpaste every day during the experiment before being stored inside a glass beaker. The tooth had been characterized by Optical Microscopy (OM) and Secondary Electronic Microscopy (SEM), before and after the experiment.
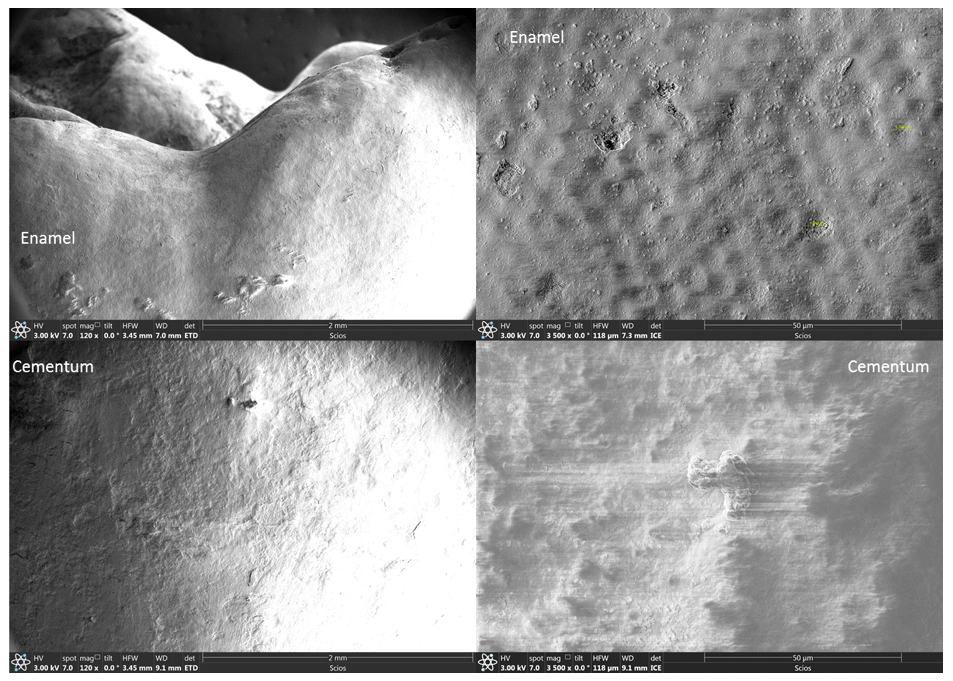
The outside of the human tooth is composed of two parts: crown covered by Enamel (Figure 1.) and root covered by Cementum (Figure 2.). The Enamel surface is smoother than Cementum. Micro-cell structures of 5~7 µm in diameter can be observed on Enamel, both in OM (Figure 1.) and SEM (Figure 3.)
Figure 1. Enamel on Crown, under Optical Microscope (OM) before the experiment.
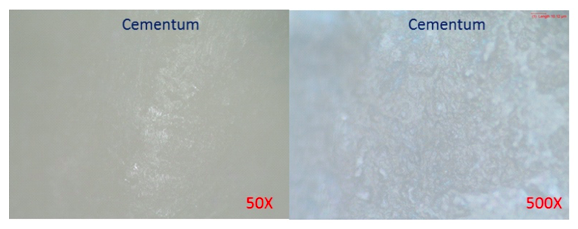
Figure 2. Cementum on Root, under Optical Microscope (OM) before the experiment.
Figure 3. Enamel & Cementum, under Secondary Electronic Microscopy (SEM) before the experiment.
Results
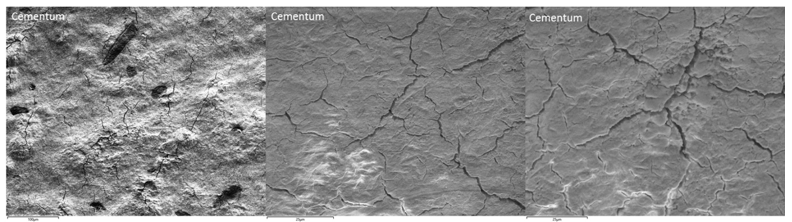
After 2 months’ immersion of the human tooth in the Rising® Denta 5000 Plus Fluoride toothpaste, the human tooth was washed clean and characterized with OM and SEM again to investigate any change on the tooth surface. The images taken are listed in the Figure 4 to 6 in the following. Notice that micro-cracks have been observed to appear on both Enamel and Cementum surfaces. Enamel surface started to show small groups of micro-cracks with stretching lengths of 50µm to 100µm (Figure 5.); while extensive micro-crack networks showed up on the surface of Cementum (Figure 6.). Obviously, the micro-crack density on the Cementum surface is a lot higher than the Enamel surface.
Discussion
The micro-cracks that appeared on the human tooth should have made the tooth weaker and more brittle. The cause of micro-cracks is understandable since there is a possibility that the Fluoride ion in the toothpaste could react and even erode the Calcium in the tooth over a long period of time.
Figure 4. Enamel & Cementum, under Optical Microscope (OM) after 2months’ experiment; Specially, cementum has shown crack networks.
Figure 5. Enamel under Secondary Electronic Microscopy (SEM) after 2months’ experiment; Enamel started to show small groups of cracks.
Figure 6. Cementum under Secondary Electronic Microscopy (SEM) after 2 months’ experiment; Extensive micro-crack networks have shown up on the surface of the Cementum.
Conclusion
Fluoride is known to be a cancer-causing mineral that is commonly found in toothpaste and in some areas’ tap water. Fluoride can be possibly dangerous at high levels where it can weaken your bones and teeth. It can also be toxic to the brain and nerve cells with enough exposure to fluoride.
In a 2-month experimental study of the long-term effect of Fluoride toothpaste on human teeth, it is observed that micro-cracks have appeared on both Enamel and Cementum surfaces, which is a sign of tooth degradation. While Cementum surface has a much higher density of micro-crack networks, it seems that Cementum is more easily eroded by Fluoride than Enamel.
This study only investigated the effects of fluoride toothpaste on human teeth over a time interval (equivalent to 47 years’ exposure) and did not include other outside influences. Factors such as chewing and the nutrients of the food we eat are some other factors that can affect the tooth’s health even further. The PH value of food residues stuck between the teeth could be another factor to affect the pace of damage caused by Fluoride toothpaste to the teeth.
*
Acknowledgement: The figure included were obtained with the help of Wei Zhou. The primary author is grateful for his help and guidance in using optical and scanning microscopy.
Sources
https://www.medicalnewstoday.com/articles/fluoride-toothpaste#what-is-fluoride
https://www.cancer.gov/about-cancer/causes-prevention/risk/myths/fluoridated-water-fact-sheet
Notes
[1] Dr. Gary Null, “Fluoride: Killing Us Softly”, Global Research, March 03, 2018
[2] Joel Griffiths, “Fluoride: Commie Plot or Capitalist Ploy,” Covert Action, Fall 1992, Vol. 42, p. 30.
[3] BETTE HILEMAN, “Fluoridation of Water- Questions about health risks and benefits remain after more than 40 years”, Chemical and Engineering News, 8/1/88, p. 36.
[4] George Glasser, “Dental Fluorosis – A Legal Time Bomb!” Sarasota/Florida ECO Report, Vol. 5, No. 2, Feb. 1995, pp. 1-5.
[5] Christa Danielson et al., “Hip fractures and fluoridation in Utah’s elderly population,” JAMA, Vol. 268, Aug. 12, 1992, pp. 746-48.
[6] “Middletown, Maryland latest city to receive toxic spill of fluoride in their drinking water,” report by Truth About Fluoride, Inc., in Townsend Letter for Doctors, 10/15/94, p. 1124.
[7] Reprinted by M. Bevis, “Morbidity associated with ingestion/dialysis of community water fluoride,” CDC, Dental Div., 6/11/92, distributed by Safe Water Foundation of Texas.
[8] John Yiamouyiannis and Dean Burk, “Fluoridation of public water systems and cancer death rates in humans,” presented at the 57th annual meeting of the American Society of Biological Chemists, and published in Fluoride, Vol. 10, No. 3, 1977, pp. 102-103.
[9] Mark Lowey, “Scientists question health risks of fluoride,” Calgary Herald, Calgary, Alberta, Canada, Feb. 28, 1992; in Griffiths, op. cit., p. 66.