How Can Moderna’s Respiratory Syncytial Virus (RSV) Jab be Approvable? 200 Side Effects (10 Severe) for each RSV Infection it Stopped
RSV is a cold for most adults. Will regulators step up?

All Global Research articles can be read in 51 languages by activating the Translate Website button below the author’s name.
To receive Global Research’s Daily Newsletter (selected articles), click here.
Click the share button above to email/forward this article to your friends and colleagues. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
***
The company’s own trial shows its shot caused 200 side effects (10 severe) for each RSV infection it stopped. You read that right: 200 to 1. RSV is a cold for most adults. Will regulators step up?
As demand for its mRNA Covid shot craters, Moderna is chasing approval for a new mRNA jab with similarly severe side effects – but against a virus even milder than Covid.
Winning approval for the jab, which reduces cases of respiratory syncytial virus, or RSV, is critical for Moderna’s future. The company has told investors RSV shots could make $10 billion a year.
The only problem: data from Moderna’s own pivotal clinical trial for its RSV shot. The trial showed the new jab causes hundreds of side effects – including 10 cases of severe side effects – for each RSV infection it prevents.
If RSV were very dangerous, that side-effect profile might make sense.
But it isn’t. The Centers for Disease Control reports that RSV usually causes “mild, cold-like symptoms.”
As it pursues approval, Moderna has vastly overestimated RSV’s lethality. A review of death certificates found that RSV kills about 35 American adults a year – about as many as die in lightning strikes, and one four-hundredth as many as Moderna has claimed. (Yes, you read that right; Moderna has overstated adult deaths from RSV by a factor of 400. Proof below.)
When Moderna applied for authorization for its Covid mRNA jab in 2020, the world was so desperate to beat the coronavirus that regulators ignored obvious red flags about side effects.
Three years later, will they make the same mistake twice?
(THE ANSWER, OR AT LEAST MORE QUESTIONS, BELOW.)
To its credit (and likely at the FDA’s insistence), Moderna ran a large and carefully designed Phase 3 clinical trial for its RSV jab.
Like its Covid cousin, the RSV shot consists of a strand of mRNA inside a tiny ball of fat, or lipid nanoparticle. The mRNA hijacks our cells so they make a protein that is part of the virus. Our immune systems then recognize that protein as an invader, giving us a headstart in making antibodies to attack the real virus.
Moderna began its Phase 3 RSV trial in February 2022 and enrolled about 35,000 adults over age 60. Half received the jab and half a placebo shot that had no mRNA. (The RSV jab requires only a single dose for its initial regimen, unlike the Covid shot.)
Eleven months later, Moderna had great news!
“mRNA-1345 demonstrated vaccine efficacy of 83.7% against RSV lower respiratory tract disease, defined by 2 or more symptoms in older adults,” the company reported in a press release on Jan. 17, 2023. And the shot was “generally well-tolerated.”
Based on those results, the company said it would submit the jab for approval.
*
Moderna wasn’t lying. Exactly.
But here’s what the trial actually showed.
Nine people who received the shot got RSV. Fifty-five who received the placebo did. Thus the 17,500 shots prevented 46 cases of RSV.
What about side effects? Moderna didn’t break out those numbers in the initial press release.
But a month later, in a respiratory virus conference in Portugal, it did. For reasons that are not clear, the presentation is difficult to find online, but it is available here.
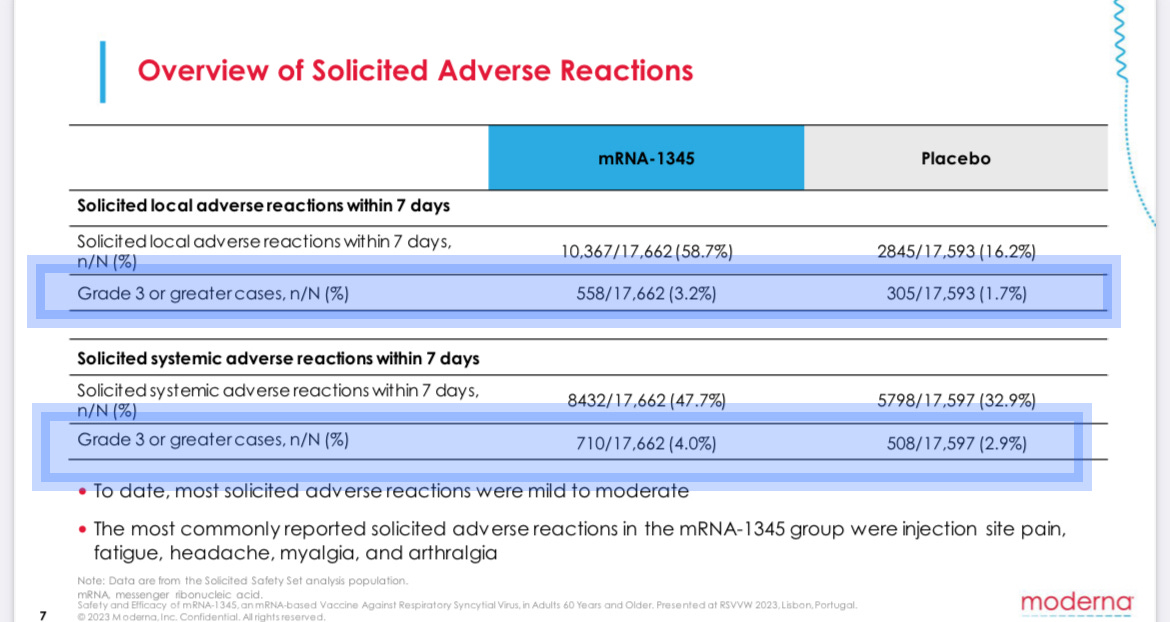
It shows people who received the jab instead of the placebo reported an extra 10,156 side effects such as headache or fatigue. Those side effects included an extra 455severe effects, rated as Grade 3 or worse.
Side effects are rated on a five-point scale, with Grade 5 being death and Grade 4 usually requiring immediate medical treatment and hospitalization. Grade 3 side effects are defined as “severe or medically significant.”
For example, a Grade 3 fever is usually defined as over about 102 degrees, while a Grade 4 is over 104.
In other words, a single Grade 3 side effect is likely to be considerably more severe than a case of RSV for most adults. Again, Moderna’s shot caused 10 of those side effects for every RSV infection it prevented.
(Whole lot of adverse reactions going on!)
Moderna also did not and has not broken out what are called “serious adverse events” in the trial.
Those are a separate category of side effect that can occur at any time in a clinical trial; regulators and investigators then judge whether they are related to the vaccine. In the trials for mRNA Covid shots, people who had received the shots had many more serious adverse events as well as immediate side effects; it is likely the RSV jabs followed the same pattern, though until Moderna discloses the data no one outside the company will know.
This terrible side effect profile might be slightly more palatable if adults needed only one RSV shot for permanent protection, but neither Moderna nor anyone else is even pretending that will be the case.
In its presentations to investors, Moderna groups the RSV shot with Covid and flu jabs, which are given annually (or more, in the case of the Covid shot). Further, both clinical trial and real-world experience with Covid shots suggests that the side effects will be the same or worse as the number of mRNA doses increases. In other words, the side effect profile Moderna disclosed in the Phase 3 trial is the best-case scenario – not the worst.
*
Two days before its presentation at the Portugal conference, Moderna tweeted an announcement about the conference, with a short video that included this factoid:
(The dead, piled like cordwood…)
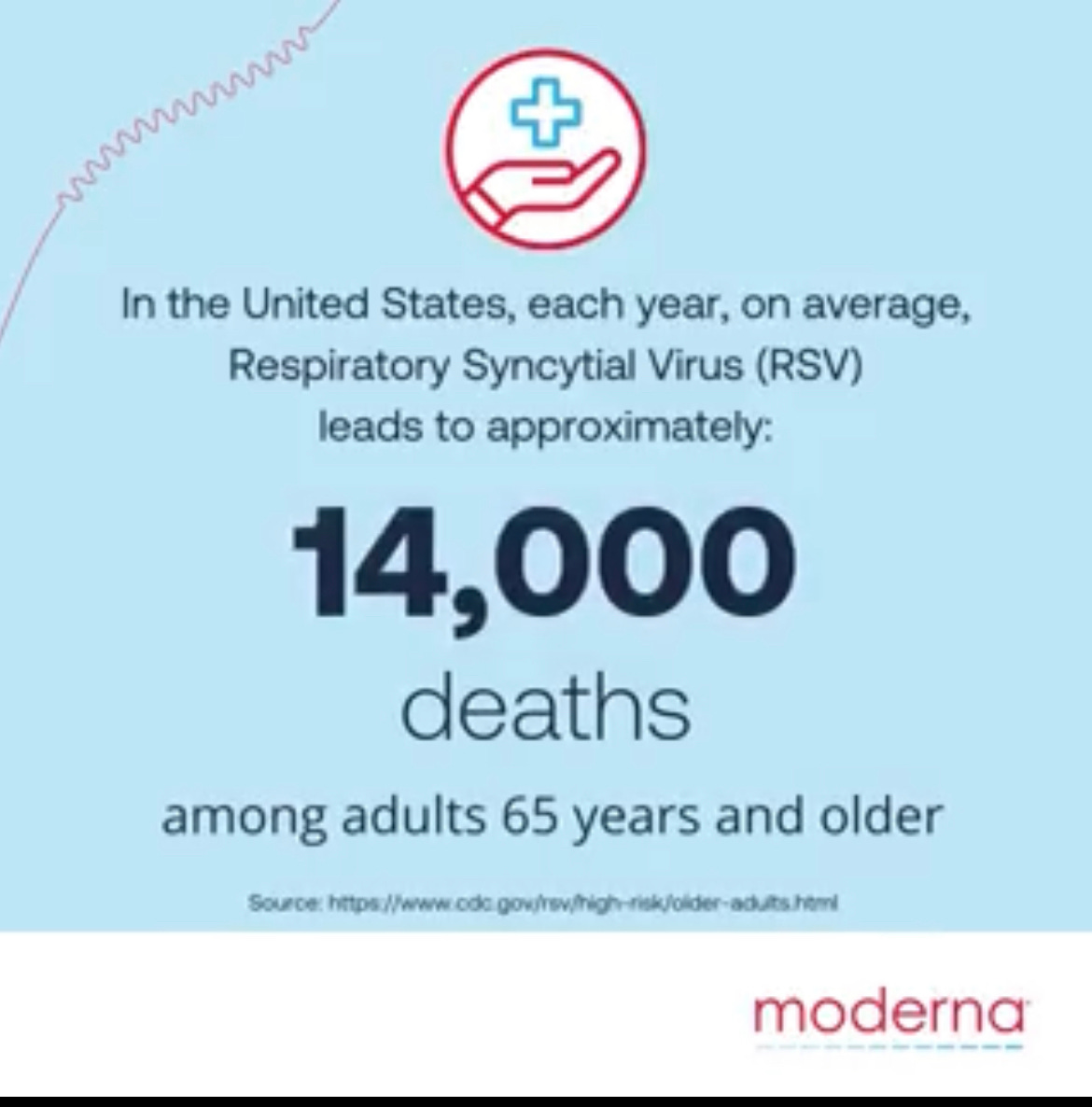
As Moderna pursues approval for the RSV shot, it has repeatedly pointed to estimates that RSV causes 10,000 or more deaths in older adults.
But those figures are based – wait for it – on computer modeling that attempts to correlate changes in RSV infections with the number of deaths from pneumonia and other respiratory illnesses.
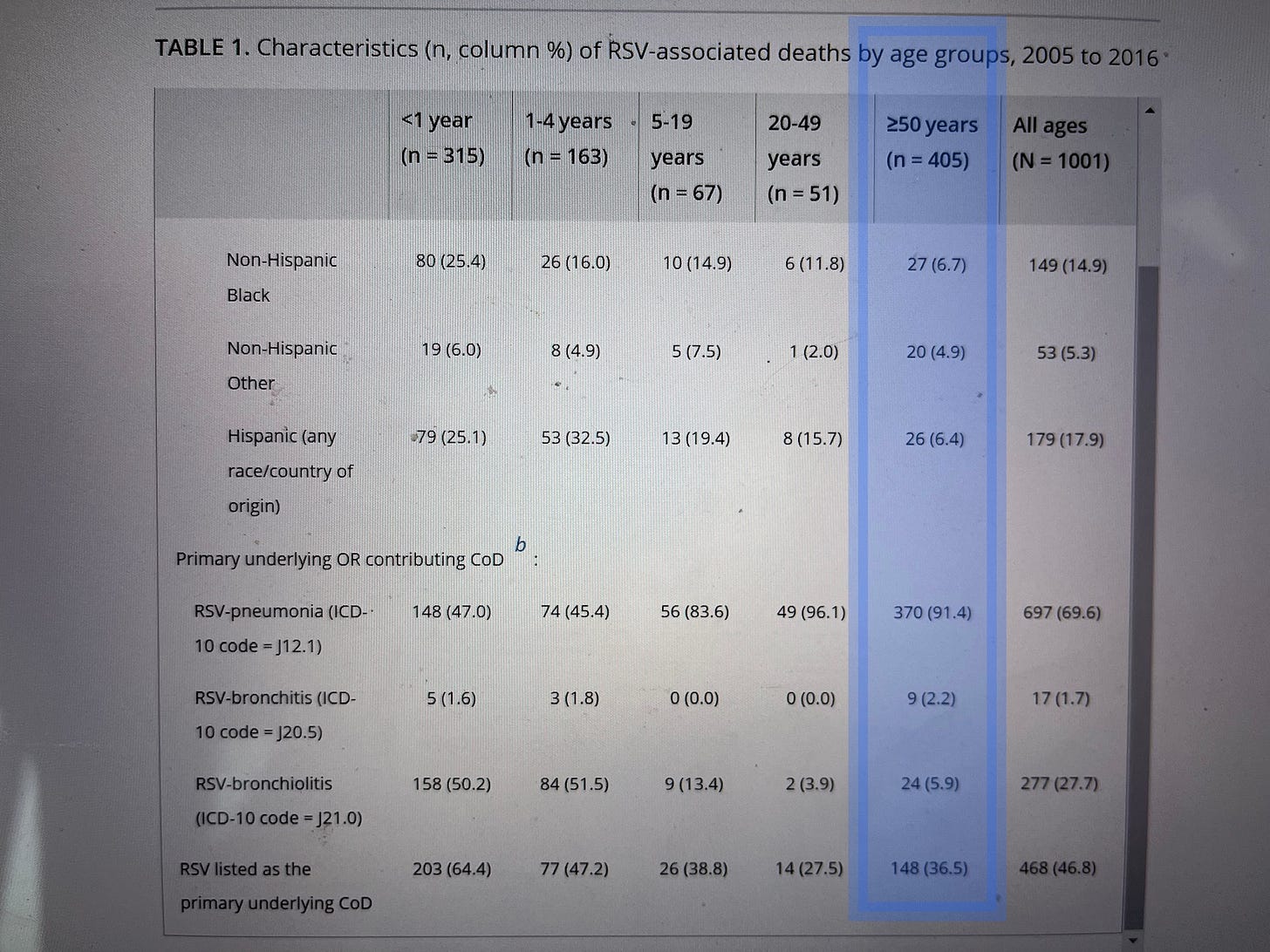
When researchers look at death certificates directly to see how physicians have codedthe cause of death, they find far, far fewer RSV deaths. In 2021, researchers examined every death certificate in the United States from 2005 to 2016 to see how many deaths had been attributed to RSV.
They found 405 deaths in adults over 50 over the twelve-year period.
That’s 35 a year. Not 14,000.
And only 148 of those cases – about 12 a year – were deaths where RSV was listed as a primary cause rather than a contributing factor.
That’s about one-sixth as many Americans as are killed by lawnmowers every year.
(Back to life, back to reality… See that “148” at the bottom of the blue highlighted column? That’s how many American adults over 50 actually died primarily from RSV. In TWELVE years.)
Still, Moderna is barreling ahead with its new shot.
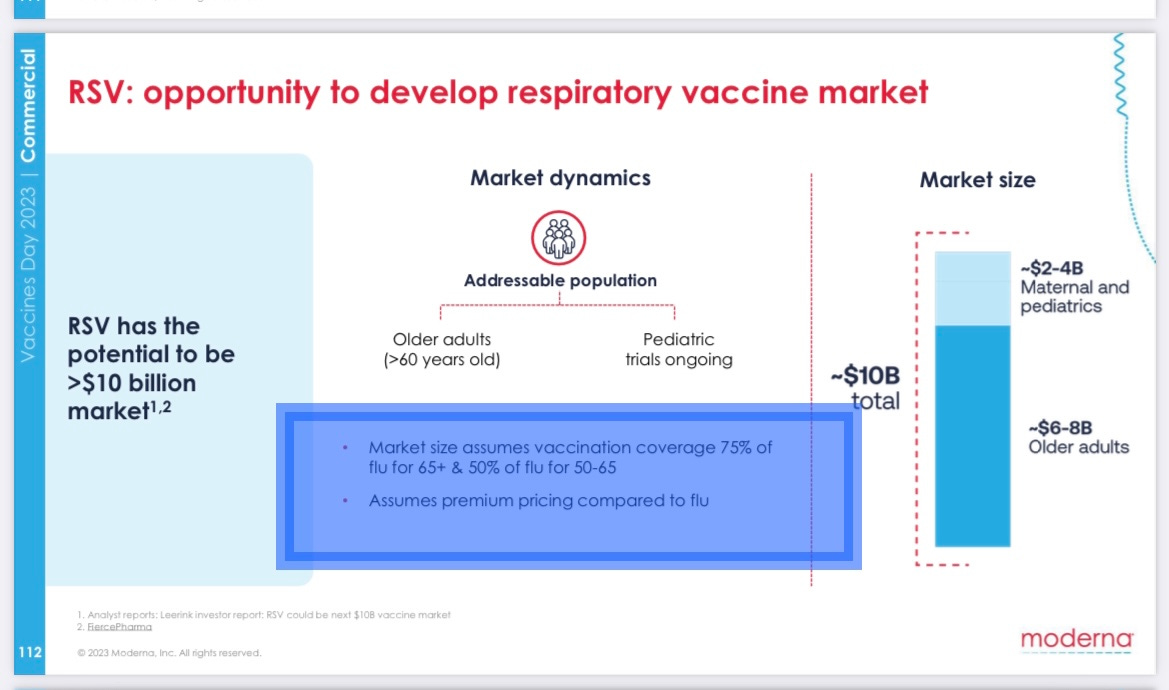
On its 4th annual “vaccines day” three weeks ago, it told investors it expected to be able to charge “premium pricing compared to flu” – even though influenza is far more dangerous than RSV to adults and flu shots have far fewer side effects.
RSV shots for adults could be an $8 billion annual market, the company claimed.
Then again, Moderna managed to make $35 billion selling Covid jabs that in the long run actually appear to increase the risk of getting Covid. Why wouldn’t it think it can handle the FDA this time around too? It has told investors to expect “potential regulatory action… as early as 4Q2023.”
Given the side effect profile of the RSV shot, Moderna’s confidence is striking. The company may simply believe regulators will be afraid to stand up to it – knowing that doing so will inevitably raise questions about the Covid jab.
For drug companies, cynicism pays. Very well indeed.
*
Note to readers: Please click the share button above. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.

