Expert Evidence Regarding Comirnaty (Pfizer) COVID-19 mRNA Vaccine for Children
All Global Research articles can be read in 51 languages by activating the “Translate Website” drop down menu on the top banner of our home page (Desktop version).
Visit and follow us on Instagram at @crg_globalresearch.
***
This expert statement was submitted by Italian lawyer Renate Holzeisen in conjunction with a lawsuit that challenges the EU’s authorization of the use of Pfizer’s mRNA vaccine on children of 12 years and older. The arguments made here specifically reference the Pfizer vaccine, but they apply similarly to the Moderna mRNA vaccine, and many also apply to the adenovector-based AstraZeneca and Johnson & Johnson vaccines.
Summary
This expertise on the use of the Pfizer COVID-19 vaccine (Comirnaty, BNT162b2) in ado- lescents is divided into three sections, which will deal with the following questions, in order:
1. Is vaccination of adolescents against COVID-19 necessary?
2. Is the Pfizer COVID-19 vaccine effective?
3. Is the Pfizer COVID-19 vaccine safe?
The arguments presented in Section 1 pertain to all COVID-19 vaccines, whereas those in Sections 2 and 3 apply specifically to the Pfizer vaccine.
Section 1 will show that vaccination of adolescents COVID-19 is unnecessary, because
- in this age group the disease is almost always mild and benign;
- for the rare clinical cases that require it, treatment is readily available;
- immunity to the disease is now widespread, due to prior infection with the virus (SARS-CoV-2) or with other coronavirus strains; and
- asymptomatic adolescents will not transmit the disease to other individuals who might be at greater risk of infection.
Section 2 will demonstrate that the claims of efficacy which Pfizer attaches to its vaccine— namely, 95% efficacy in adults, and 100% in adolescents—are
- misleading,becausethesenumberspertaintorelative,notabsoluteefficacy,thelatter being on the order of only 1%;
- specious, because they refer to an arbitrarily defined, clinically meaningless eval- uation endpoint, whereas no efficacy at all has been demonstrated against severe disease or mortality;
- most likely altogether fraudulent.
Section 3 will show that the safety profile of the Pfizer vaccine is catastrophically bad. It will be discussed that
- Pfizer, the EMA, and the FDA have systematically neglected evidence from preclinical animal trials that clearly pointed to grave dangers of adverse events;
- the Pfizer vaccine has caused thousands of deaths within five months of its introduction;
- The agencies that granted emergency use authorization for this vaccine committed grave errors and omissions in their assessments of known and possible health risks.
The only possible conclusion from this analysis is that the use of this vaccine in adolescents cannot be permitted, and that its ongoing use in any and all age groups ought to be stopped immediately.
1 Vaccination of adolescents against COVID-19 is unnecessary
1.1 What does the available evidence show? There are several lines of evidence that show vaccination of adolescents against COVID-19 to be unnecessary.
1.1.1 The case fatality rate of COVID-19 in the general population is low. The vast majority of all persons infected with COVID-19 recovers after minor, often uncharacteristic illness. According to world-leading epidemiologist John Ioannidis [1, 2], the infection fatality rate of COVID-19 is on the order of 0.15% to 0.2% across all age groups, with a very strong bias towards old people, particularly those with co-morbidities. This rate does not exceed the range commonly observed with influenza, against which a vaccination of adolescents is not considered urgent or necessary.
1.1.2 COVID-19 has a particularly low prevalence and severity in adolescents. In the U.S. and as of April 2020, those younger than 18 years accounted for just 1.7% of all COVID-19 cases [3, 4]. Within this age group, the most severe cases were observed among very young infants [4]. This is consistent with the lack in infants of cross-immunity to COVID-19, which in other age groups is conferred by preceding exposure to regular respi- ratory human coronaviruses (see Section 1.2.1). Among slightly older children, a peculiar multisystem inflammatory syndrome was observed in early 2020 [5]; conceivably, these patients, too, were still lacking cross-immunity.
Essentially no severe cases of COVID-19 were observed in those above 10 but below 18 years of age [4]. This group accounted for just 1% of reported cases, almost all of which were very mild. Thus, adolescents are at particularly low risk of harm from COVID-19 infection. Vaccination of this age group is therefore unnecessary.
1.1.3 COVID-19 can be treated. Numerous experienced physicians have collaborated on establishing effective treatment guidelines for clinically manifest COVID-19 [6]. Treatment options are available both for the early stage of the disease, at which emphasis is placed on inhibiting viral replication, and for the later stage, at which anti-inflammatory treatment is paramount. Two drugs that have been used successfully at the early stage are hydroxychloroquine and ivermectin. Both drugs have been, and continue to be, in use against a variety of other diseases. Ivermectin, for example, is considered safe enough to be used not only for treating manifest scabies—a parasite infection of the skin that is unpleasant but not severe—but even prophylactically in asymptomatic contacts of scabies-infected persons [7].
Ivermectin is also widely used in the treatment of tropical parasitic diseases such as onchocerciasis (river blindness), and for this reason it is on the WHO’s list of essential medicines. Yet, with COVID-19, the WHO sees fit to warn against the use of this very same well-known and safe drug outside of clinical trials [8]. This policy cannot be rationally justified, and it has quite appropriately been overridden by national or regional health authorities and ignored by individual physicians worldwide.
The availability of effective treatment voids the rationale for the emergency use of vaccines on any and all age groups, including also adolescents.
1.1.4 Most people, particularly adolescents, are by now immune to SARS-CoV-2. Due to the many inherent flaws and shortcomings of the diagnostic methods in common use (see Section 1.2), it is impossible to accurately determine the proportions of those who have already been infected with SARS-CoV-2 and those who have not. However, there are indications that the proportion of those who have been infected and recovered is high:
- The incidence of multisystem inflammatory syndrome in children (see Section 1.1.2) peaked in early to mid 2020, and then receded, with some slight delay after the initial wave of the COVID-19 respiratory disease itself [9].
- Approximately 60% of randomly selected test persons from British Columbia have detectable antibodies against multiple SARS-CoV-2 proteins (personal communication by Stephen Pelech, University of British Columbia), indicating past infection with the virus—as opposed to vaccination, which would induce antibodies to only one (the spike) protein.
Past COVID-19 infection has been found to protect very reliably from reinfection [10], and strong specific humoral and cellular immunity is detected in almost all recovered individuals, and also in those who remained asymptomatic throughout the infection [11]. Thus, a large proportion of individuals in all age groups, including adolescents, already have specific, reliable immunity to COVID-19. As mentioned above, most of those who do not have such specific immunity nevertheless are protected from severe disease by cross- immunity [12, 13]. This immunity will be particularly effective in healthy adolescents and young adults. Individuals with specific immunity or sufficient cross-immunity cannot possibly derive any benefit from undergoing an experimental vaccination.
1.1.5 Asymptomatic transmission of COVID-19 is not real. An oft-cited rationale for vaccinating individuals who are not themselves at risk of severe disease is the need to induce “herd immunity:” the few who are at high risk should be protected by preventing the spread of the virus in the general population.
A subtext of this rationale is the idea of “asymptomatic spread”—persons who have been infected but who show no signs of it other than a positive PCR test are assumed to transmit this infection to other susceptible individuals. If we accept the idea of such asymptomatic spread, then preventative mass vaccination might indeed appear as the only means of reliable protection of those at risk.
It has, however, been unambiguously determined that such asymptomatic transmission does not occur. In a large-scale study, which involved almost 10 million Chinese residents, no new infections could be traced to persons that had tested positive for SARS- CoV-2 by PCR, but who did not exhibit any other signs of infection [14]. This agrees with several studies that compared PCR to virus isolation in cell culture among patients with acute COVID-19 disease. In all cases, growth of the virus in cell culture ceased as symptoms subsided, or very shortly thereafter, whereas PCR remained positive for weeks or months afterwards [15, 16]. It was accordingly proposed to use cell culture rather than PCR to assess infectiousness and to determine the duration of isolation [16].
These findings indicate that restricting contact of persons at risk with those who show, or very recently showed, symptoms of acute respiratory disease would be effective and sufficient as a protective measure. Indiscriminate mass vaccinations of persons who are not themselves at risk of severe disease are therefore not required to achieve such protection.
1.2 Missing evidence: use of inaccurate diagnostic methods. A key element that is lacking in the current discussion of the need for vaccination is a reliable diagnostic tool for determining who is or is not currently infected with SARS-CoV-2. The diagnostic procedure most widely used for this purpose is based on the polymerase chain reaction (PCR). The PCR is a very powerful and versatile method that lends itself to numerous ap- plications in molecular biology, and also in the laboratory diagnosis of viral infections. However, exactly because it is so powerful, PCR is very difficult to get right even at the best of times; it will yield accurate results only in the hands of highly trained and disci- plined personnel. The enormous scale on which the method has been deployed during the COVID-19 pandemic has meant that it was entrusted to untrained and insufficiently supervised personnel; in such circumstances, the mass manufacture of false-positive re- sults due to the cross-contamination of samples is a disaster waiting to happen (see for example [17]). While this alone already is reason for grave concern, the problems start even earlier—namely, with the design of the PCR tests and the guidelines used for their interpretation, which would lead to false positive results even in the hands of skilled and diligent workers.
The key conclusion from this section will be that the PCR tests which have been used throughout the pandemic, and which continue to be used, lack accuracy and specificity and cannot be relied on for diagnostic or epidemiological purposes. In order to ade- quately justify these conclusions, we must first consider the basics of the method in some detail.
1.2.1 Coronaviruses and SARS-CoV-2. Coronaviruses are a large family of enveloped, positive strand RNA viruses. In humans and a variety of animal species, they cause res- piratory tract infections that can range from mild to lethal in severity. The vast majority of coronavirus infections in humans cause mild illness (common cold), although in very young children, who lack immunity from previous exposure, respiratory disease can be more severe. Note that the same clinical picture is also caused by viruses from several other families, predominantly rhinoviruses. Three clinical syndromes—SARS, MERS, and COVID-19—are associated with specific coronavirus strains that have “emerged” only within the last 20 years.
The virus that causes COVID-19 is known as Severe acute respiratory syndrome coro- navirus 2 (SARS-CoV-2). The World Health Organization (WHO) declared the outbreak a Public Health Emergency of International Concern on January 30th, 2020, and a pandemic on March 11th, 2020. While it has been maintained that SARS-CoV-2 arose naturally in a species of bats [18], a thorough analysis of the genome sequences of SARS-CoV-2 and of related virus strains indicates unambiguously that the virus is in fact of artificial ori- gin [19–22]. Initially decried as a “conspiracy theory,” this explanation has recently and belatedly been gaining acceptance in the mainstream.
1.2.2 The polymerase chain reaction. The polymerase chain reaction (PCR) is a ver- satile method for the biochemical replication of deoxyribonucleic acid (DNA) in vitro. Immediately after its invention by Kary Mullis in the 1980s, PCR took the world of molecular biology by storm, finding application for creating DNA mutations, DNA sequencing, for shuffling and merging nucleic acids of different origin (recombinant DNA technology), and for the creation of novel nucleic acids or even whole genomes from scratch (“synthetic biology”). PCR also soon found its way into the field of diagnostic medical microbiology [23]. Particularly with respect to viral pathogens, PCR is now one of the mainstay diagnostic methods. Against this background, it is not surprising that PCR methods should also have been adopted in the laboratory diagnostics of SARS-CoV-2.
1.2.2.1 The principle. To understand how PCR works, it is best to start with a piece of double-stranded DNA (the well-known double helix). In such a molecule, each of the paired single strands consists of four different building blocks (nucleotides), which will here be referred to as A, C, G, and T for short. Within each single strand, these building blocks are arranged like pearls on a string; the biological activity and identity of the nucleic acid will be dictated by its characteristic nucleotide sequence.
In a DNA double helix, the two strands are held together by the proper pairing of the nucleotides, such that an A in one strand is always found opposite to a T in the other, and likewise C is always found opposite G. Thus, the nucleotide sequence of one strand implies that of the other—the two sequences are complementary.
The first step in PCR consists in the separation of the two strands, which can be ef- fected by heating the DNA sample past its “melting point.” Each strand can now be used as a template for synthesizing a new copy of its opposite strand. To this end, two short, synthetic single-stranded DNA molecules (“primers”) are added; their sequences are cho- sen such that one will bind to each of the DNA template strands, based on sequence complementarity. For this binding to occur, the temperature of the reaction must be lowered.
Once the primers have bound, each is extended by the repeated incorporation of free nucleotide precursors to one of its two free ends. This is accomplished using a thermostable DNA polymerase—a bacterial enzyme that synthesizes DNA. The extension is carried out at a temperature which is intermediate between those used for double strand separation and primer binding (“annealing”). After this step has extended each of the primers into a new DNA strand, we will have created two double-stranded DNA molecules from one. We can now repeat the process—separate the two double strands and convert them into four, then eight, and so on. After 10 cycles, the initial amount of double-stranded DNA will have increased by a factor of approximately one thousand, after 20 cycles by a million, and so on—amplification proceeds exponentially with the number of reaction cycles, until the reaction finally runs out of primers and/or nucleotide precursors.
1.2.2.2 PCR and RNA templates. While the above discussion referred to DNA only, PCR can also be used with RNA templates; this is important with SARS-CoV-2, since this virus has RNA rather than DNA as its genetic material. To this end, the RNA is first converted (“reversely transcribed”) into DNA, using a reverse transcriptase enzyme. The DNA copy of the viral RNA genome is referred to as complementary DNA (cDNA).
1.2.3 Potential pitfalls of PCR in diagnostic applications. We just saw that PCR allows us to take a very small sample of DNA and amplify it with extraordinary efficiency. How- ever, this very efficiency of amplification creates a number of problems that must be carefully addressed in order to make the result meaningful, particularly in a diagnostic context.
1. If we use too high a number of repeated reaction cycles, minuscule amounts of nucleic acids will be detected that have no diagnostic significance.
2. The various temperatures used in the reaction must be carefully calibrated, and they must match the length and nucleotide sequence of the two DNA primers. If in particular the temperature for primer annealing is too low, then the primers may bind to the template DNA in a non-specific manner—in spite of one or more mismatched nucleotides—and DNA molecules other than the intended ones may be amplified. In the context of COVID diagnostics, this could mean that for example the nucleic acids of coronaviruses other than SARS-CoV-2 are amplified and mistaken for the latter.
3. Apart from the temperature, other conditions must likewise be carefully calibrated in order to ensure specificity. These include in particular the concentrations of magne- sium ions and of free nucleotides; excessively high concentrations favour non-specific amplification.
There is a further problem that results not from the efficiency of the amplification, but rather from a technical limitation: PCR is most efficient if the amplified DNA molecule is no more than several hundred nucleotides in length; however, a full-length coronavirus genome is approximately 30,000 nucleotides long. Successful amplification of a segment of several hundred nucleotides only thus does not prove that the template nucleic acid itself was indeed complete and intact, and therefore that it was part of an infectious virus particle.
1.2.4 Technical precautions in diagnostic PCR. Non-specific or overly sensitive ampli- fication can be guarded against in a number of ways:
- All primers that are part of the same reaction mixture must be designed in such a manner that they anneal to their template DNA at the same temperature. As may be intuitively clear, a longer primer will begin to anneal to its template at a higher temperature than a shorter one; and since the bond which forms between C and G on opposite strands is tighter than that between A and T, the nucleotide composition of each primer must also be taken into account. If the primers are mismatched in this regard, then the more avidly binding primer will start to bind non-specifically when the temperature is low enough for allowing the other primer to bind specifically. The original Corman-Drosten PCR protocol [24] that was rapidly endorsed by the WHO has been criticized for exactly this mistake [25].
- Instead of amplifying only a single piece of the template DNA, one can simultaneously amplify several pieces, using the appropriate number of DNA primer pairs, and stipu- late that all pieces, or a suitable minimal number, must be successfully amplified for the test to evaluate as positive.
- One must keep track of the “cycle threshold” or Ct value for short, that is, the num- ber of amplification cycles that were necessary to produce a detectable amount of amplified product; the lower the number of cycles, the greater the initial amount of template nucleic acid that must have been present.
- Confirming the identity—the exact nucleotide sequence—of the nucleic acid mole- cules that were amplified. DNA sequencing has been feasible in diagnostic routine laboratories for a considerable time, and there is no good reason not to use it, partic- ularly when decisions pertaining to public health depend on these laboratory results.
1.2.5 Real-time PCR. The third point above, and to a degree the fourth, can be ad- dressed using real-time PCR. In this method, the accumulation of amplified DNA is moni- tored as the reaction progresses, in real time, with product quantification after each cycle (quantitative PCR; qPCR for short). Real-time detection can be achieved by the inclusion of a third DNA primer, which binds to either of the template DNA strands, at a location between the two other primers which drive the DNA synthesis. Downstream of the binding of that third primer, a light signal will be emitted, and the intensity of this signal is proportional to the amount of amplified DNA present. Since binding of this primer, too, requires a complementary target sequence on the DNA template, this method does provide some confirmation of the nucleotide sequence of the target DNA.
A second, simpler variety of real-time PCR uses a simple organic dye molecule that binds to double-stranded DNA. The dye displays weak background fluorescence that increases dramatically upon DNA binding. The measured fluorescence increase is then proportional to the total amount of amplified DNA; but since the dye binds regardless of DNA sequence, in this case the signal does not give evidence that the correct template DNA has been amplified.
1.2.6 Shortcomings of commercial COVID-19 PCR tests. Unfortunately, the number of amplification cycles (the Ct value) needed to find the genetic material in question is rarely included in the results sent to authorities, doctors and those tested. Most commercially available RT-qPCR tests set the limit of amplification cycles up to which an amplification signal should be considered positive at 35 or higher. Multiple studies have indicated that Ct values above 30 have a very low predictive value for positive virus cultures, and thus for infectiousness or the presence of acute disease [15, 26–28]. Considering that in many clinical trials—including the ones conducted by Pfizer (see later)—a “COVID-19 case”, or an “endpoint” amounts to no more than a positive PCR test, regardless of Ct value, in combination with one or a few non-specific symptoms of respiratory disease, the significance of the use of improperly high Ct cut-off values cannot be overstated. This systematic and widespread error alone has sufficed to gravely distort the diagnoses conferred on individual patients, as well as the epidemiology of the pandemic as a whole.
Further systematic negligence concerns the verification of the identity of the ampli- fied DNA fragments. While Sanger DNA sequencing of such fragments, the gold standard, is feasible on a large scale in principle, it has not been routinely used in the ongoing mass PCR testing campaigns. The error is compounded by the very low number of independent PCR amplifications considered sufficient for a positive test—as few as two, or even only one have been considered sufficient in various jurisdictions—as well as by various other technical faults in the widely adopted and commercialized Corman-Drosten protocol, which have been discussed in detail elsewhere [25].
In summary, a positive RT-qPCR test result cannot be accepted as proof that the per- son in question is currently infected and infectious—even if there is reasonable clinical plausibility of actual COVID-19 infection, as well as a significant community prevalence of the disease. Firstly, the RNA material containing the target sequences could very well be from nonviable/inactive virus; this is particularly likely if the patient in question has already recovered from the infection. Secondly, there needs to be a minimum amount of viable virus for onward transmission; but tests carried out with excessively high (yet unreported) Ct values will detect minuscule amounts of genetic material that pose no real risk at all.
2 The Pfizer COVID-19 vaccine lacks efficacy
2.1 What does the evidence show? Pfizer persistently touts the 95% efficacy of its vaccine, based on the clinical trials that formed the basis of the emergency approvals granted by the FDA [29] and the European Union [30]. In a more recent study on adolescents [31], the claimed efficacy has been raised to no less than 100%. However, these claims cannot be taken at face value.
2.1.1 Absolute vs. relative efficacy. In Pfizer/BioNTech’s first reported clinical trial, 43,548 participants underwent randomization, of whom 43,448 received injections. The experimental vaccine (BNT162b2) was administered to 21,720 persons, and 21,728 re- ceived placebo. Across both groups, a total of 170 COVID-19 “cases” was recorded, of which 162 occurred in the placebo group, whereas 8 cases were observed in the BNT162b2 group. Based on these figures—8/162 ≈ 5%—Pfizer proceeded to claim 95% effi- cacy. Clearly, however, this efficacy is only a relative value—in absolute terms, less than 1% of the placebo group developed COVID-19, and therefore less than 1% of the vaccine group was protected from it.
The situation is similar with the subsequent, smaller test carried out on 12-15 years old adolescents [31]. Here, the vaccine group comprised 1131 individuals, whereas the placebo group included 1129 persons. In the latter group, 16 individuals were subse- quently diagnosed with COVID-19, whereas no such cases occurred in the vaccine group. True to form, Pfizer/BioNTech converted this absolute efficacy of 1.4% to a relative one of 100%; only the latter value is highlighted in the abstract of the published study.
2.1.2 Negative impact of BNT162b2 on overall morbidity in adolescents. In the cited vaccine study on adolescents, a “case” of COVID-19 was determined as follows:
The definition of confirmed COVID-19 included the presence of ≥ 1 symptom (i.e., fever, new or increased cough, new or increased shortness of breath, chills, new or increased muscle pain, new loss of taste or smell, sore throat, diarrhea, vomiting) and being SARS-CoV-2 NAAT-positive [= PCR-positive] dur- ing, or within 4 days before or after, the symptomatic period (either at the central laboratory or at a local testing facility and using an acceptable test).
Thus, a single symptom from a laundry list of non-characteristic symptoms, plus a positive finding from an unreliable laboratory test (cf. Section 1.2.6), was deemed suffi- cient to establish the diagnosis. While the study goes on to list several clinical criteria of severe disease, it gives no indication that any test persons actually suffered any of those. It can therefore be assumed that very few non-severe, and no clinically severe cases of COVID-19 occurred in the entire test population.
In stark contrast to these numbers pertaining to the disease from which the vaccina- tion is supposed to protect, side effects from the vaccination were exceedingly common. Apart from injection site pain occurring in a high percentage of the vaccine group (79% to 86%), fatigue (60% to 66%) and headache (55% to 65%) abounded. Severe fatigue and headache were reported by several percent of the test persons. Severe headache, in par- ticular, may be associated with underlying thrombotic events (see Section 3.1.3.2). It is therefore clear that, if we consider both COVID-19 and vaccine adverse effects, overall morbidity was far greater in the vaccinated than in the placebo group.
2.1.3 Unlikely claims and contradictions in Pfizer’s evidence on efficacy. We saw above that the reported efficacy of Pfizer’s vaccine is very modest when expressed in absolute terms. Even this low efficacy, however, cannot be accepted at face value. This is apparent from the assessment reports prepared by the FDA [29] and the EMA [30].
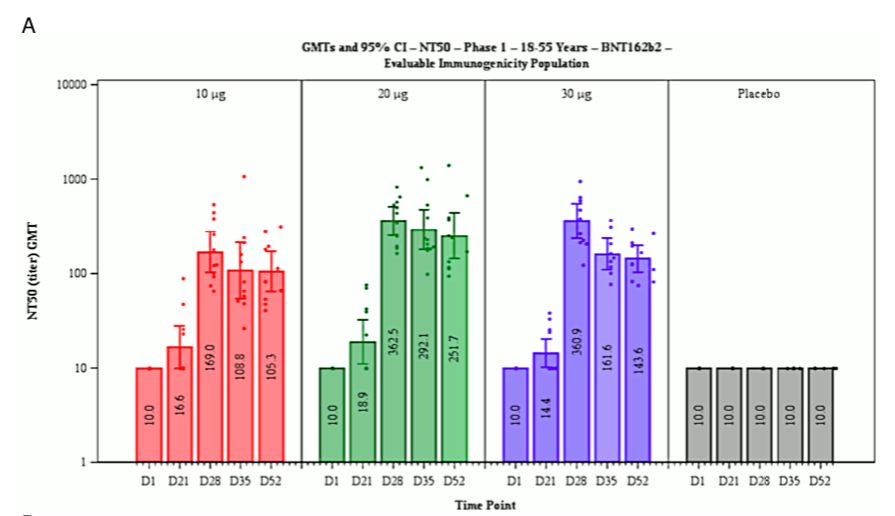
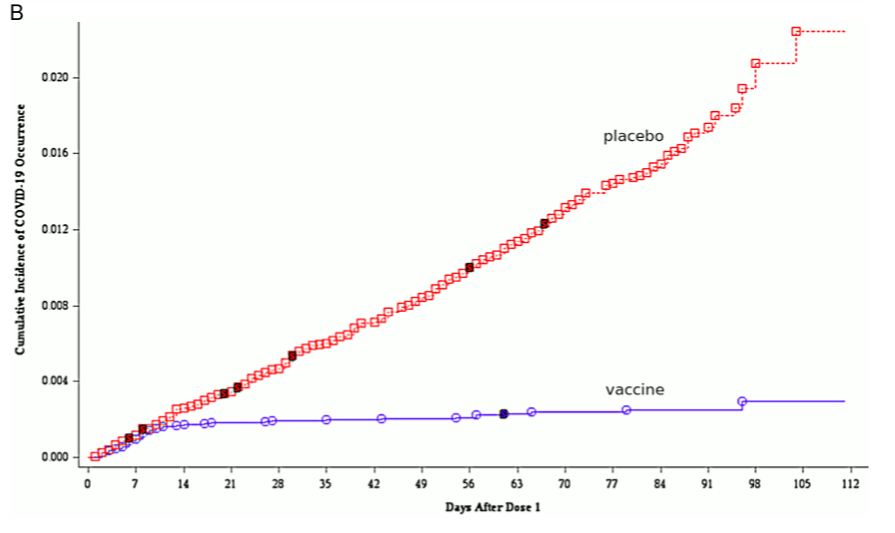
2.1.3.1 Sudden onset of immunity on day 12 after the first injection. A key illustration that occurs in both reports compares the cumulative incidence of COVID-19 among the vaccinated and the placebo group. This graph, which is shown as Figure 9 in the EMA report, is here reproduced in Figure 1B. Up to day 12 after the first injection, the cumulative incidences in the two groups track each other closely. After day 12, however, only the placebo group continues to accumulate further new cases at a steady pace, whereas the slope of the graph drops to almost zero in the vaccine group.
This remarkable observation suggests that immunity sets in very suddenly and uni- formly on day 12 exactly among the vaccinated. Since the second injection occurred 19 or more days after the first one, this would imply that one injection is enough to estab- lish full immunity. This conclusion, however, is not stated, and in fact Pfizer does not report any data at all on test persons who received one injection only.
A sudden onset of full immunity on day 12 after the first exposure to the antigen is not at all a biologically plausible outcome. Typically, immunity develops more slowly and gradually; and such a pattern is in fact reported for this very same vaccine (BNT162b2) in Figure 7 of the EMA report, reproduced here as Figure 1A. The figure shows the increase of neutralizing antibodies to SARS-CoV-2 as a function of time after the first injection of the vaccine.
Figure 1 Reproduction of Figure 7 (A; neutralizing antibody titres on various days after the first injection) and of Figure 9 (B; cumulative incidence of COVID-19 among vaccinated and placebo groups) from the EMA assessment report [30]. Note the logarithmic y axis in B. See text for discussion.
Table 1 Subjects without evidence of infection in vaccine and placebo groups at various time points in the clinical trial. Data excerpted from Table 4 in [30]. See text for discussion.
The induction of neutralizing antibodies is the declared purpose of the Pfizer vaccine. Generally speaking, antibodies are protein molecules produced by our immune system when it encounters antigens—macromolecules that do not occur within our own bodies. These antigens are often part of infectious microbes, including viruses. An antibody binds to a specific feature on the surface of its antigen; this feature is called the epitope of the antibody in question.
In the context of virus infections, antibodies can be neutralizing or non-neutralizing. A neutralizing antibody recognizes an epitope that is essential for the function of the virus, for example because this epitope must make contact to a receptor molecule on the surface of the host cell which the virus must enter in order to replicate. A non- neutralizing antibody simply happens to recognize a surface feature (epitope) that plays no essential role in the infectiousness of the virus.
Considering the foregoing, we should expect that the blood level of neutralizing antibodies should reflect the degree of clinical immunity to the virus. This is, however, not at all what we see in Figure 1A. On day 21 after the first injection, that is, a full 9 days after the purported sudden onset of full clinical immunity, the amount of neutralizing antibodies in the blood has barely risen above the background level. The maximal level of neutralizing antibodies is observed only on day 28 after the first injection, at which time most test persons would already have had their second injection. The time course of cellular (T-cell) immunity was not reported, but in the absence of proof positive to the opposite it can be assumed to resemble that of the antibody response.
It is very difficult to reconcile the two contrasting observations of sudden onset of full clinical immunity on day 12, but neutralizing antibodies appearing only weeks later. Yet, neither the EMA reviewers nor those of the FDA appear to have been interested in the problem.
2.1.3.2 The Pfizer documentation contradicts itself on COVID-19 incidence after vaccination.
Table 1 lists the percentages of subjects in the vaccine group and the placebo group who showed no evidence of SARS-CoV-2 infection on day 0 (before the first dose) and on day 14 after the second dose, respectively. From the differences between the two time points, we can work out that 7.5% of the subjects in the vaccine group and 8% in the control group converted from negative to positive—that is, became infected—between the two time points.
According to [29], the second dose was administered approximately 21 days after the first, although all subjects who received it between days 19 and 42 after the first injection were included in the evaluation. If we take day 35 after the first injection as the approximate time point of the comparison, we see from Figure 1B that the cumulative incidence between day 0 and day 35 is more than twice higher in the placebo group than in the vaccine group; but from Table 1, we see that it is almost the same. Moreover, with both groups the numbers are substantially higher in the table than in the figure.
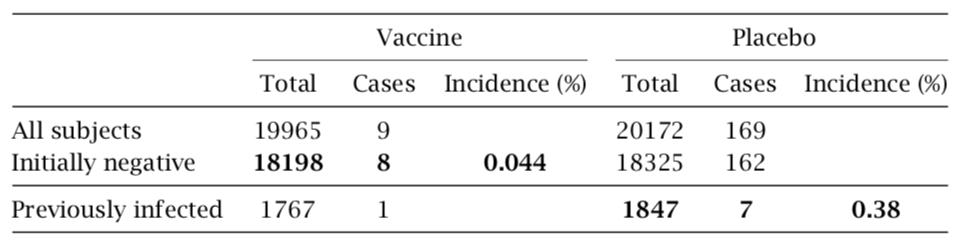
Table 2 Incidence of COVID-19 among subjects not previously infected but vaccinated, or previously infected but not vaccinated. Data excerpted from Tables 6 and 7 in [29]. See text for discussion.
These two sets of data cannot possibly be reconciled; one must be false. Since, as discussed, the sudden onset of immunity implied by Figure 1B lacks any biological plau- sibility, it is most likely that it is this data set which was fabricated.
2.1.3.3 Pfizer’s data imply that the vaccine protects from COVID more effectively than does prior infection with the virus. We can also scrutinize Pfizer’s reported data in order to compare the immunity conferred by the vaccine to that induced by prior natural infection with the virus. The relevant data are summarized in Table 2. The reported 8 cases of COVID-19 among vaccinated persons who had initially tested negative for the virus amount to an incidence of 0.044%. Pfizer also reports 7 cases among persons who had initially tested positive but were not vaccinated. Since this group is considerably smaller, those 7 cases translate into an almost ninefold higher incidence (0.38%).
It is common knowledge that vaccines will at best approach, but not surpass the im- munity conferred by the corresponding natural infection. Very robust immunity after prior natural infection with SARS-CoV-2 has recently been reported [10]; in that study, not a single case of COVID-19 was observed among 1359 individuals who had remained unvaccinated. Robust immunity after infection is also confirmed by comprehensive lab- oratory investigations [11]. Therefore, the above analysis corroborates yet again that the trial results reported by Pfizer cannot be trusted. That neither the FDA nor the EMA picked up on any of these inconsistencies does not instil confidence in the thoroughness and integrity of their review processes.
2.2 What evidence is lacking to make the case? We had already mentioned the specious and contrived character of the endpoint used in Pfizer’s clinical trials—namely, the count- ing of a COVID-19 “case” based on nothing more than a positive PCR result, together with one or more items from a list of mostly uncharacteristic clinical symptoms. We must therefore ask if the vaccine provides any benefits that are more substantial than the claimed—but, as discussed above, most likely fabricated—reduction in the count of such trivial “cases.”
2.2.1 Prevention of severe disease and mortality. Page 48 of the FDA report sums up this question as follows: “A larger number of individuals at high risk of COVID-19 and higher attack rates would be needed to confirm efficacy of the vaccine against mortality.”
We note that this quote not only answers the posed question in the negative, but it also disposes of the entire pretext for granting emergency use authorization for this experimental vaccine. If in a study that involves 40,000 individuals the number of fatal outcomes is too small to permit the detection of any benefit of the vaccine, then surely no “emergency” exists that would justify the very grave risks, and meanwhile manifest harm, associated with the extraordinarily rushed introduction of this and other COVID- 19 vaccines.
No fatalities at all occurred in the cited study on adolescents [31]; and we already noted that this study does not report any cases of severe disease either. Therefore, in this specific age group, too, neither a meaningful benefit nor an emergency are in evidence.
2.2.2 Effectiveness for those at high-risk of severe COVID-19. Here, the FDA report has this to say: “Although the proportion of participants at high risk of severe COVID- 19 is adequate for the overall evaluation of safety in the available follow-up period, the subset of certain groups such as immunocompromised individuals (e.g., those with HIV/AIDS) is too small to evaluate efficacy outcomes.”
The report shirks the question of risk reduction among those with more common predisposing conditions, such as for example chronic heart or lung disease. Naturally, the clinical study on adolescents [31] is completely barren in this regard. Overall, no evidence has been adduced by Pfizer’s clinical studies to prove clinical benefit in those at high risk of severe COVID-19.
2.2.3 Effectiveness against long-term effects of COVID-19 disease. The FDA report’s verdict is as follows: “Additional evaluations will be needed to assess the effect of the vaccine in preventing long-term effects of COVID-19, including data from clinical trials and from the vaccine’s use post authorization.” In other words, the clinical trials pro- vided no such evidence.
2.2.4 Reduction of transmission. On this topic, the FDA report offers only that “addi- tional evaluations including data from clinical trials and from vaccine use post-autho- rization will be needed to assess the effect of the vaccine in preventing virus shedding and transmission, in particular in individuals with asymptomatic infection.”
In plain language, there is no evidence that transmission is reduced, and in fact the trials were simply not even designed to prove or disprove such an effect.
2.2.5 Duration of protection. The FDA report correctly states (on page 46) that “as the interim and final analyses have a limited length of follow-up, it is not possible to assess sustained efficacy over a period longer than 2 months.” Even if we choose to believe that any efficacy at all has been demonstrated pertaining to the two-month study period, such a short duration of protection does not justify the risks associated with vaccination.
2.2.6 Inadequate efforts to determine the optimal dose. Figure 1A shows that the level of neutralizing antibodies is virtually the same with vaccine (mRNA) doses of 20μg and 30μg, respectively. This raises the question why the higher dose was employed throughout—and not only with adults, on whom these data were obtained, but also with children, whose lower body weights should suggest a dose reduction. Furthermore, the data in Figure 1B suggest that full immunity is induced already by the first dose; appli- cation of the second dose does not change the pace at which new cases accrue in the vaccine group, and therefore apparently has no effect on immunity. This would imply that a one-dose regimen should have been evaluated, which would reduce the overall likelihood of adverse events.
2.2.7 Summary. The clinical trials carried out by Pfizer contain no proof of any benefit conferred by the vaccine with respect to any clinically relevant endpoints. This applies to all tested age groups, and in particular also to adolescents.
3 The Pfizer COVID-19 vaccine lacks safety
3.1 What does the evidence show? The clinical trials for Comirnaty (BNT162b2), as well as for the other COVID-19 vaccines, were rushed through in a very short time; this has meant that proper precautions to ensure their safety were not taken. However, animal experiments carried out before the start of clinical testing already gave reason to expect severe toxicity. Unfortunately, this expectation has been abundantly borne out in practice since the beginning of mass vaccinations.
3.1.1 Preclinical data from animal experiments indicate potential for grave harm.
Comirnaty, like all other gene-based COVID-19 vaccines, causes the expression in vivo of one specific protein of SARS-CoV-2—namely, the so-called spike protein, which is lo- cated on the surface of the virus particle. The spike protein mediates the virus particle’s initial attachment to the host cell and also its subsequent entry into the cell. The key idea behind the Comirnaty vaccine is as follows:
- a synthetic mRNA that encodes the spike protein is complexed with a mixture of neutral and cationic (positively charged) synthetic lipids, which cluster together in lipid nanoparticles (LNPs);
- after injection, the LNPs facilitate the uptake of the mRNA into host cells, where the mRNA will cause the expression (synthesis) of the spike protein;
- the spike protein will appear on the surface of the host cells and induce an immune reaction to itself.The immune reaction to the spike protein will comprise both antibodies, which may or may not be neutralizing (see Section 2.1.3.1), and T-lymphocytes (T-cells). Some of these T-cells are cytotoxic (also known as T-killer cells); their function is to kill virus- infected body cells.
While this vaccination strategy may look good on paper, it has a number of drawbacks and risks. These arise both from the lipid mixture and from the spike protein, both of which have known toxic activities.
3.1.1.1 Toxic and procoagulant activities of the spike protein. Severe clinical COVID- 19 disease is often accompanied by a pathological activation of blood clotting [32]. The central role of the spike protein in this complication is recognized [33]. Notably, there are at least two different mechanisms for triggering blood coagulation:
- If the spike protein is expressed within vascular endothelial cells—the innermost cell layer of the blood vessels—then an immune reaction to the spike protein can destroy these cells. The resulting vascular lesion will activate blood clotting. This immune reaction can involve cytotoxic T-cells, but also antibodies that trigger the complement system and other immune effector mechanisms.
- Spike protein molecules that are formed within the circulation, or which enter it after being synthesized elsewhere in the body, can directly bind to blood platelets (thromboycytes) and activate them. This will again set off blood clotting.
The second mechanism is significant because it does not involve an immune reaction; therefore, it can be triggered right away even in those persons who have no pre-existing immunity. The first mechanism will be most effective in those who already have immunity to the spike protein, due to either infection with the virus or a previous injection of vaccine. Note that the underlying mechanism of cell damage will also operate in other tissues—any cell in the body that expresses the spike protein will thereby become a tar- get for the immune system.
Since Comirnaty and other gene-based vaccines induce the synthesis of active, and therefore potentially toxic, spike protein, it is important to understand how this protein with be distributed within the body. Toxicity might be limited if the vaccine, and there- fore the synthesis of the spike protein, remained confined to the site of injection, within the muscle tissue but outside the circulation. On the other hand, if the vaccine were to enter the bloodstream, then one would have to expect expression of the spike protein within the blood vessels and toxicity through the activation of blood clotting.
3.1.1.2 Distribution of the vaccine in animal experiments. As it turns out, the vac- cine does indeed appear in the bloodstream very rapidly after intramuscular injection. In experiments which Pfizer reported to the Japanese health authorities [34], rats were injected with a mock vaccine sample. This material was was chemically similar to Comir- naty, but it contained an mRNA molecule that encoded an easily traceable, non-toxic model protein (luciferase) rather than the SARS-CoV-2 spike protein. The lipid mixture used to form the LNPs was the exact same as with Comirnaty. One of the lipids in this mixture was radioactively labelled, which permitted the distribution of the sample within the body to be traced and quantified sensitively and accurately. Several remarkable ob- servations were made:
- The radioactive lipid appeared rapidly in the bloodstream. The blood plasma concen- tration peaked after 2 hours; but even at only 15 minutes into the experiment, the plasma level had already reached 45% of that maximal value.
- Very high levels of the radioactive lipid accumulated in the liver, the spleen, the adrenal glands, and the ovaries.
- Comparatively low levels accumulated in the central nervous system (the brain and the spinal cord).
- Expression of the model protein encoded by the mRNA was studied only in the liver, where it was readily detected.
3.1.1.3 Mechanism of vaccine uptake into the bloodstream. Considering that the com- plex consisting of mRNA with bound LNPs has a rather large molecular size, we must ask how it managed to enter the bloodstream so rapidly. After intramuscular injection, the bulk of the vaccine should end up in the “interstitial” space, that is, the extracellular space outside the blood vessels. This space is separated from the intravascular space (the circulation) by the capillary barrier, which permits free passage only to small mo- lecules such as oxygen or glucose (blood sugar) but is impermeable to large molecules such as plasma proteins; and the vaccine particles would be even larger than those.
The fluid within the interstitial space is continuously drained through the lymphatic system; all lymph fluid ultimately enters the bloodstream through the thoracic duct. Par- ticles which are too large for traversing the capillary barrier can ultimately reach the circulation by way of this lymphatic drainage. However, this process tends to be consid- erably slower [35] than was observed here with the model vaccine. We must therefore ask if the model vaccine may have broken down the capillary barrier and thereby gained direct entry to the bloodstream.
Lipid mixtures similar to those contained in the Pfizer vaccine have been used exper- imentally to penetrate the blood brain barrier after intravenous injection [36]. The blood brain barrier can be described as a “fortified version” of the regular capillary barrier—if it can be broken down, then we must expect the same with a regular capillary barrier, too. The high local concentration of the lipid nanoparticles that will result after intra- muscular injection will further promote the breakdown of the barrier. The upshot of this is that the vaccine will appear in the bloodstream, in large amounts and on short order. Complications due to blood clotting must therefore be expected.
3.1.1.4 Other indications of LNP toxicity. The proposed breakdown of the capillary barrier by the LNPs implies a cytotoxic effect on the endothelial cells, which form the only cellular element of the capillary walls. Cytotoxic effects of the LNPs are also evident from damage to muscle fibres at the injection site [30, p. 49] and to liver cells [30, p. 46]. Note that these data, too, were obtained with the model mRNA encoding the presumably non-toxic luciferase enzyme. Therefore, these cytotoxic actions are not due to any direct action of the spike protein. An immunological component of the cell damage cannot be completely ruled out, but it is likely not dominant in this case, since luciferase, unlike spike protein, is not transported to the cell surface.
3.1.1.5 Mechanisms of accumulation in specific organs. The high rates of accumulation of the vaccine in the liver and the spleen suggest uptake by macrophage cells, which abound in both organs and are generally in charge of clearing away unwanted de- bris. The accumulation in the adrenal glands, the ovaries, and again the liver suggests a role of lipoproteins in cellular uptake within these organs. Lipoproteins are complexes of lipids and specific protein molecules (apolipoproteins) that function as lipid carriers in the bloodstream. The liver has a central role in lipid and lipoprotein metabolism generally, whereas the adrenal glands and the ovaries take up lipoproteins to acquire cholesterol, which they then convert to their respective steroid hormones. Such a role of lipoproteins in the transport and cellular uptake of lipid nanoparticles is in fact accepted [37]. We must therefore expect that other organs with a high rate of lipoprotein uptake will be similarly affected. This includes in particular the placenta, which like the ovaries produces large amounts of steroid hormone (progesterone), and the lactating mammary glands, which acquire cholesterol contained in lipoproteins for secretion into the breast milk.
3.1.1.6 Correlation of lipid uptake and mRNA expression. In the experimental study in question, the liver was also shown to express the mRNA that is associated with the LNPs (see [30], Section 2.3.2). As stated above, the mRNA used in this study encoded the firefly enzyme luciferase, which is the very protein that enables these animals to glow in the dark. Mammalian tissues expressing this enzyme will also become luminescent, in proportion to the amount of luciferase protein which they synthesize. Measurements of this luminescence are not very sensitive, though, which was most likely the reason why Pfizer carried them out only with the liver but not with other, smaller organs. However, in the absence of proof positive to the opposite, we must assume that the correlation between efficient LNP uptake and mRNA expression that applies to the liver will also hold with other organs. If the cargo mRNA encodes the spike protein, then these organs will be exposed to the toxicity of the spike protein, and to the immune reaction against it, in proportion to the level of LNP and mRNA uptake.
3.1.1.7 Potential risks to fertility and to the breastfed newborn. A high level of expression of spike in the ovaries raises the prospect of significant damage to that organ, with possible consequences for female fertility. Uptake of the vaccine by mammary gland cells opens two possible pathways of toxicity to the breastfed child: firstly, the expression of spike protein and its secretion into the breast milk, and secondly, the wholesale transfer of the vaccine into the milk. The mammary glands are apocrine, which means that they pinch off and release fragments of their own cytoplasm into the milk; thus, anything that has reached the cytoplasm might also reach the breast milk. In this connection, we note that both the VAERS database and the EU drug adverse events registry (EudraVigilance) report fatalities in breastfed newborns after vaccination of their mothers (see Section 3.1.3.6).
3.1.1.8 Pfizer’s failure to investigate risks evident from preclinical investigations.
With the exception of fertility, which can simply not be evaluated within the short period of time for which the vaccines have been in use, all of the risks discussed above have been substantiated since the vaccines have been rolled out—all are manifest in the re- ports to the various adverse event registries (see Section 3.1.3). We must stress again that each of these risks could readily be inferred from the cited limited preclinical data, but were not followed up with appropriate in-depth investigations. In particular, the clinical trials did not monitor any laboratory parameters that could have provided information on these risks, such as those related to blood coagulation (e.g. D-dimers/thrombocytes) or liver damage (e.g. γ-glutamyltransferase).
3.1.2 Contaminations arising from the manufacturing process. The commercial scale manufacturing process of BNT162b2 gives rise to several contaminations that may com- promise vaccine safety and effectiveness. For brevity, we will here mention only two such contaminants.
3.1.2.1 Contaminating bacterial DNA. The mRNA is produced in vitro using a DNA template, which in turn is obtained from bacterial cells. While steps are taken to remove this DNA afterwards, they are not completely effective, which is acknowledged in the EMA report (pages 17 and 40). Contaminating DNA injected with the vaccine may insert into the genomes of host cells and cause potentially harmful mutations. Bacterial DNA also non-specifically promotes inflammation.
3.1.2.2 Lipid impurites. The EMA report also observes impurities originating from the synthesis of the lipid ingredients of the vaccine (page 24):
Lipid-related impurities have been observed in some recently manufactured finished product batches, correlated with ALC-0315 lipid batches. The quality of ALC-0315 excipient is considered acceptable based on the available data on condition that specific impurities in the finished product will be further evaluated.
Considering that the synthetic lipid referred to as ALC-0315 has never before been used on humans, there is no sound empirical basis for deciding on “acceptable” levels of impurities. Furthermore, it appears that the contaminating species have not even been identified. EMA’s arbitrary blanket approval of unknown contaminants of an unproven vaccine ingredient is completely unacceptable.
3.1.3 Adverse events after the onset of vaccinations. Since the introduction of the vaccines, numerous adverse events have been reported to registries around the world. We will here focus on two registries, namely, the U.S. vaccine adverse events reporting system (VAERS) and the EU monitoring system for drug adverse events (EudraVigilance). All numbers quoted below are as of May 21st unless stated otherwise.
3.1.3.1 Fatalities reported in connection with COVID vaccines. Within just five months of the onset of vaccinations, EudraVigilance has accumulated 12,886 deaths in connection with the COVID-19 vaccines, of which the Pfizer vaccine accounted for almost half (6,306). In the same time period, VAERS has run up 4,406 deaths in all; of these, 91% were associated with the mRNA vaccines, with Pfizer accounting for 44% and Moderna for 47% of the total.
It is impossible to know what percentage of all fatalities that occur after vaccina- tion will actually be reported to VAERS or EudraVigilance. However, note that the 4,406 COVID vaccine-related fatalities accrued by VAERS during just the past 5 months exceed the cumulative total of all other vaccines combined, over the entire previous 20 years. It is therefore clear that these vaccines are far and away the most deadly ones in history— quite predictably so, and all for a disease whose case fatality rate does not exceed that of influenza [1, 38].
3.1.3.2 Severe events related to disrupted blood clotting. The litany of diagnoses in both databases that indicate pathological activation of blood clotting is almost endless— heart attacks, strokes, thromboses in the brain and in other organs, pulmonary em- bolism; but also thrombocytopenia and bleeding, which result from excessive consump- tion of thrombocytes and of coagulation factors in disseminated intravascular coagula- tion. These disease mechanisms caused many of the fatalities summarized above; in other cases, they caused severe acute disease, which will in many cases leave behind severe disability.
3.1.3.3 Other severe reactions. Severe reactions also include seizures, other neurolog- ical symptoms, particularly related to motor control, and severe systemic inflammation with damage to multiple organs. Again, in many of these patients, long-lasting or even permanent residual damage is highly likely.
3.1.3.4 Severe adverse reactions among adolescents. In the age group of 12-17 years, two deaths likely related to the Pfizer vaccine were already reported to EudraVigilance. Also in this age group, there were 16 cases of myocarditis, all in males, and 28 cases of seizures among both sexes, 3 of them reported as life-threatening. There also were a few cases of stroke, myocardial infarction, and severe inflammatory disease.
While the numbers of adverse events are much lower than those among adults, this is simply due to the hitherto far lower rates of vaccination in this age group. Should systematic vaccination be green-lighted for adolescents, we must expect these numbers to rapidly climb to a level resembling that seen in adults.
3.1.3.5 Miscarriages. As of June 21st, 2021, EudraVigilance lists 325 cases of miscar- riage among vaccinated pregnant women. While it is difficult to ascertain by just how much vaccination will raise the rate of miscarriage, most of these cases were reported by healthcare professionals, who evidently considered a connection to the vaccine at least plausible. This series of cases alone would be reason enough to pause the vaccinations and investigate.
3.1.3.6 Deaths among breastfed infants. Although it does not directly relate to the age group which is the focus of this lawsuit and this expert opinion, it bears mention that both VAERS and EudraVigilance contain reports of death among breastfed children shortly after their mothers had received the Pfizer vaccine.
In Section 3.1.1.5, we discussed the possibility of vaccine uptake into the placenta and the breast glands. The reported miscarriages and fatalities in newborns indicate that these risks must be taken very seriously, and that Pfizer acted negligently in not investigating them in any of their reported preclinical and clinical trials.
3.2 Missing evidence. We saw above that significant positive indications of risk were neglected in the clinical trials and subsequent rushed emergency approval of the Pfizer vaccine, with unfortunate yet predictable outcomes. Equally damning is the list of omissi- ons—potential risks that should have been investigated in preclinical or clinical trials but never were.
3.2.1 Proper pharmacokinetics. Section 3.1.1.2 described some experiments pertaining to the distribution of a surrogate vaccine. While these studies did provide important and useful information, it must be noted that the expression of the spike protein instead of the presumably inert luciferase enzyme might affect the distribution due to its interfer- ence with vascular integrity, including at the blood brain barrier, and with blood clotting. EMA and other regulators should have insisted that such experiments be carried out and documented.
3.2.2 Drug interactions. The EMA report states (page 110): Interaction studies with other vaccines have not been performed, which is acceptable given the need to use the vaccine in an emergency situation.
Since it is clear that mortality due to COVID-19 is low (see Section 1.1.1) and therefore that no emergency exists, this argument must be rejected as specious.
Immunosuppressive effects of BNT162b2 are apparent from a drop of blood lym- phocyte numbers among those vaccinated, as well as from clinical observations of Her- pes zoster (shingles), which arises through the reactivation of persistent varicella-zoster virus [39]. This suggests that the desired immune response to other vaccines simultane- ously administered may be impaired.
Furthermore, studies of interactions should not have been limited to vaccines alone, but also been extended to other drugs. One area of concern is the experimentally ap- parent liver toxicity of BNT162b2. The liver is central in the metabolic inactivation and disposal of many drugs; any interference with the function of this organ immediately creates numerous possibilities of adverse drug interactions.
3.2.3 Genotoxicity. No studies have been carried out regarding genotoxicity, that is, damage to the human genetic material, which could lead to heritable mutations and cancer. In the EMA report [30, p. 50], this is justified as follows:
No genotoxicity studies have been provided. This is acceptable because the components of the vaccine formulation are lipids and RNA, which are not expected to have genotoxic potential. The risk assessment performed by the ap- plicant shows that the risk of genotoxicity related to these excipients [i.e. the synthetic lipids] is very low based on literature data.
In reality, it is known that the LNPs contained in BNT162b2 can enter all kinds of cells—that is, after all, the purpose of their inclusion in this vaccine preparation. It is also known that, once inside the cell, cationic lipids disrupt mitochondrial function (cell respiration) and cause oxidative stress, which in turn leads to DNA damage.
It should be mentioned that two of the lipids used by Pfizer—namely, the cationic lipid ALC-0315 and the PEGylated lipid ALC-0159, which account for 30-50% and for 2- 6%, respectively, of the total lipid content—had not previously been approved for use in humans. Pfizer’s and EMA’s cavalier attitude to the use of novel and so far unproven chemicals as components in drug or vaccine preparations without comprehensive studies on toxicity, including genotoxcicity, is completely unscientific and unacceptable.
3.2.4 Reproductive toxicity. Reproductive toxicity was assessed using only one species (rats) and on only small numbers of animals (21 litters). A greater than twofold increase in pre-implantation loss of embryos was noted, with a rate of 9.77% in the vaccine group, compared to 4.09% in the control group. Instead of merely stating [30, p. 50] that the higher value was “within historical control data range,” the study should have stated un- ambiguously whether or not this difference was statistically significant; and if it was not, the number of experiments should have been increased to ensure the required statistical power. The same applies to the observations of “very low incidence of gastroschisis, mouth/jaw malformations, right sided aortic arch, and cervical vertebrae abnormalities.” Overall, these studies are inadequately described and apparently were also inadequately carried out.
3.2.5 Autoimmunity. Exposure to the vaccine will lead to cell damage due to the cationic lipids, and also to the immune attack on cells producing the spike protein. From the cells undergoing destruction, proteins and other macromolecules will be released; such mate- rial must then be cleared away by macrophages.
When the clearing system is overloaded because of excessive cell damage and apoptosis (cell death), then the accumulation of cellular debris will lead to chronically excessive type I interferon release; this, in turn, will trigger further inflammation. With time, some macromolecules in the debris will become targets for the formation of autoanti- bodies and the activation of autoreactive cytotoxic T cells—they will begin to function as auto-antigens. This then leads to further tissue damage and the release of more auto- antigens—autoimmune disease will develop. Such an outcome is particularly likely in im- munocompromised people or in those who are genetically predisposed to autoimmune disease (e.g. those with the HLA-B27 allele).
The risk of autoimmunity induced by BNT162b2 could be adequately addressed only in long-term studies; as with fertility or cancer, the very short period of preclinical and clinical testing means that we are flying blind. It should go without saying that all of these risks are particularly grave with children, adolescents, and young adults.
3.2.6 Antibody-dependent enhancement. While antibodies in principle serve to protect us from infections, in some cases they can increase disease severity. This phenomenon is referred to as antibody-dependent enhancement.
3.2.6.1 The principle. In Section 2.1.3.1 above, we saw that antibodies may or may not neutralize the virus that elicited them. While in most cases non-neutralizing antibodies are not harmful, with some viruses they can actually make matters worse by facilitating entry of these viruses into host cells. This occurs because certain cells of the immune system are supposed to take up antibody-tagged microbes and destroy them. If a virus particle to which antibodies have bound is taken up by such a cell but then manages to evade destruction, then it may instead start to multiply within this cell. Overall, the antibody will then have enhanced the replication of the virus. Clinically, this antibody- dependent enhancement (ADE) can cause a hyperinflammatory response (a “cytokine storm”) that will amplify the damage to our lungs, liver and other organs of our body.
ADE can occur both after natural infection and after vaccination, and it has been observed with several virus families, including Dengue virus, Ebola virus, respiratory syncytial virus (RSV), and HIV [40]. Importantly, ADE also occurs with coronaviruses, and in particular with SARS, whose causative agent is closely related to SARS-CoV-2. Attempts to develop vaccines to SARS repeatedly failed due to ADE—the vaccines did induce antibodies, but when the vaccinated animals were subsequently challenged with the virus, they became more ill than the unvaccinated controls (see e.g. [41]).
3.2.6.2 SARS-CoV-2 and ADE. The possibility of ADE in the context of natural infection with SARS-CoV-2, as well as of vaccination against it, has been acknowledged [42]. More specifically, ADE due to spike protein antibodies elicited by other coronavirus strains has been invoked to account for the peculiar geographical distribution of disease severity within China [43]. However, the experimental research required to address it remains missing, even after more than one year into the pandemic.
With some experimental SARS vaccines, ADE could be mitigated through the use of inulin-based adjuvants [44]. This approach might be feasible for avoiding ADE with COVID-19 vaccines also, but so far this appears not to have been investigated with any of the existing COVID vaccines.
Pfizer and the regulatory bodies are well aware of the risk of ADE as well. The FDA notes in its briefing document [29, p. 44]:
Pfizer submitted a Pharmacovigilance Plan (PVP) to monitor safety concerns that could be associated with Pfizer-BioNTech COVID-19 Vaccine. The Sponsor identified vaccine-associated enhanced disease including vaccine-associated enhanced respiratory disease as an important potential risk.
Here, the term “vaccine-associated enhanced disease” refers to ADE. EMA has likewise acknowledged that this risk must be investigated further [30, p. 141]:
Any important potential risks that may be specific to vaccination for COVID- 19 (e.g. vaccine associated enhanced respiratory disease) should be taken into account. The Applicant has included VAED/VAERD as an important potential risk and will further investigate it in the ongoing pivotal study and a post- authorization safety study.
Overall, it is clear that the risk of ADE is recognized in theory but is not addressed in practice. Given the abundant evidence of ADE with experimental SARS vaccines, this is completely irresponsible.
*
Note to readers: Please click the share buttons above or below. Follow us on Instagram, @crg_globalresearch. Forward this article to your email lists. Crosspost on your blog site, internet forums. etc.
Michael Palmer MD is Associate Professor in the Department of Chemistry at the University of Waterloo, Ontario, Canada. He studied Medicine and Medical Microbiology in Germany and has taught Biochemistry since 2001 in Canada. His focus is on Pharmacology, metabolism, biological membranes and computer programming, with an experimental research focus on bacterial toxins and antibiotics (Daptomycin). He has written a textbook on Biochemical Pharmacology.
Sucharit Bhakdi MD is Professor Emeritus of Medical Microbiology and Immunology and Former Chair at the Institute of Medical Microbiology and Hygiene, Johannes Gutenberg University of Mainz.
Stefan Hockertz is Professor of Toxicology and Pharmacology, a European registered Toxicologist and Specialist in Immunology and Immunotoxicology. He is CEO of tpi consult GmbH.
All three are founding signatories of Doctors for Covid Ethics
Notes
- [1] J. P. A. Ioannidis: Infection fatality rate of COVID-19 inferred from seroprevalence data. Bull. World Health Organ. (2020), BLT.20.265892. url: https://www.who.int/bulletin/ online_first/BLT.20.265892.pdf.
- [2] J. P. A. Ioannidis: Reconciling estimates of global spread and infection fatality rates of COVID-19: An overview of systematic evaluations. Eur. J. Clin. Invest. 5 (2021), e133554. pmid: 33768536.
- [3] CDC COVID-19 Response Team: Coronavirus Disease 2019 in Children – United States, February 12-April 2, 2020. MMWR. Morbidity and mortality weekly report 69 (2020), 422– 426. pmid: 32271728.
- [4] S. Tsabouri et al.: Risk Factors for Severity in Children with Coronavirus Disease 2019: A Comprehensive Literature Review. Pediatric clinics of North America 68 (2021), 321–338. pmid: 33228941.
- [5] J. Y. Abrams et al.: Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. J. Pediatr. 226 (2020), 45– 54. pmid: 32768466.
- [6] P. A. McCullough et al.: Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Reviews in cardiovascular medicine 21 (2020), 517–530. pmid: 33387997.
- [7] C. Bernigaud et al.: Oral ivermectin for a scabies outbreak in a long-term care facility: po- tential value in preventing COVID-19 and associated mortality. Br. J. Dermatol. 184 (2021), 1207–1209. pmid: 33454964.
- [8] Anonymous: WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. 2021. url: https://www.who.int/news-room/feature-stories/detail/who- advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical- trials.
- [9] J. Flood et al.: Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS): Prospective, national surveillance, United Kingdom and Ireland, 2020. The Lancet regional health. Europe 3 (2021), 100075. pmid: 34027512.
- [10] N. K. Shrestha et al.: Necessity of COVID-19 vaccination in previously infected individuals. medRxiv (2021). doi: 10.1101/2021.06.01.21258176.
- [11] S. S. Nielsen et al.: SARS-CoV-2 elicits robust adaptive immune responses regardless of disease severity. EBioMedicine 68 (2021), 103410. pmid: 34098342.
- [12] A. Grifoni et al.: Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181 (2020), 1489–1501.e15. pmid: 32473127.
- [13] N. Le Bert et al.: SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584 (2020), 457–462. pmid: 32668444.
- [14] S. Cao et al.: Post-lockdown SARS-CoV-2 nucleic acid screening in nearly ten million resi- dents of Wuhan, China. Nat. Commun. 11 (2020), 5917. pmid: 33219229.
- [15] R. Wölfel et al.: Virological assessment of hospitalized patients with COVID-2019. Nature 581 (2020), 465–469. pmid: 32235945.
- [16] K. Basile et al.: Cell-based culture of SARS-CoV-2 informs infectivity and safe de-isolation assessments during COVID-19. Clin. Infect. Dis. (2020). pmid: 33098412.
- [17] Anonymous: Covid: Secret filming exposes contamination risk at test results lab. 2021. url: https://www.bbc.com/news/uk-56556806.
- [18] K. G. Andersen et al.: The proximal origin of SARS-CoV-2. Nat. Med. 26 (2020), 450–452. doi: 10.1038/s41591-020-0820-9.
- [19] B. Sørensen et al.: Biovacc-19: A Candidate Vaccine for Covid-19 (SARS-CoV-2) Developed from Analysis of its General Method of Action for Infectivity. QRB Discovery 1 (2020). doi: 10.1017/qrd.2020.8.
- [20] B. Sørensen et al.: The evidence which suggests that this is no naturally evolved virus. Preprint (2020). url: https : / / www . minervanett . no / files / 2020 / 07 / 13 / TheEvidenceNoNaturalEvol.pdf.
- [21] L. Yan et al.: Unusual Features of the SARS-CoV-2 Genome Suggesting Sophisticated Labora- tory Modification Rather Than Natural Evolution and Delineation of Its Probable Synthetic Route. Preprint (2020). doi: 10.5281/zenodo.4028829.
- [22] L. Yan et al.: SARS-CoV-2 Is an Unrestricted Bioweapon: A Truth Revealed through Uncov- ering a Large-Scale, Organized Scientific Fraud. Preprint (2020). doi: 10.5281/zenodo. 4073131.
- [23] S. Yang and R. E. Rothman: PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 4 (2004), 337–48. pmid: 15172342.
- [24] V. M. Corman et al.: Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 25 (2020). pmid: 31992387.
- [25] Anonymous: Corman-Drosten review report. 2020. url: https://cormandrostenreview. com/.
- [26] R. Jaafar et al.: Correlation Between 3790 Quantitative Polymerase Chain Reaction-Positives Samples and Positive Cell Cultures, Including 1941 Severe Acute Respiratory Syndrome Coronavirus 2 Isolates. Clin. Infect. Dis. 72 (2020), e921. pmid: 32986798.
- [27] F. M. Liotti et al.: Assessment of SARS-CoV-2 RNA Test Results Among Patients Who Re- covered From COVID-19 With Prior Negative Results. JAMA internal medicine 181 (2020), 702–704. pmid: 33180119.
- [28] J. Bullard et al.: Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples. Clin. Infect. Dis. 71 (2020), 2663–2666. pmid: 32442256.
- [29] Anonymous: FDA briefing document: Pfizer-BioNTech COVID-19 Vaccine. 2020. url: https: //www.fda.gov/media/144245/download.
- [30] Anonymous: Assessment report/Comirnaty. 2021. url: https://www.ema.europa.eu/ en/documents/assessment-report/comirnaty-epar-public-assessment-report_ en.pdf.
- [31] R. W. Frenck et al.: Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N. Engl. J. Med. (2021). pmid: 34043894.
- [32] R. A. Campbell et al.: Comparison of the coagulopathies associated with COVID-19 and sep- sis. Research and practice in thrombosis and haemostasis 5 (2021), e12525. pmid: 34027292.
- [33] G. H. Frydman et al.: The Potential Role of Coagulation Factor Xa in the Pathophysiology of COVID-19: A Role for Anticoagulants as Multimodal Therapeutic Agents. TH open : com- panion journal to thrombosis and haemostasis 4 (2020), e288–e299. pmid: 33043235.
- [34] Anonymous: SARS-CoV-2 mRNA Vaccine (BNT162, PF-07302048) 2.6.4 [Summary statement of the pharmacokinetic study] (Japanese). 2020. url: https://www.pmda.go.jp/drugs/ 2021/P20210212001/672212000_30300AMX00231_I100_1.pdf.
- [35] I. C. Kourtis et al.: Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS One 8 (2013), e61646. pmid: 23626707.
- [36] C. Ye et al.: Co-delivery of GOLPH3 siRNA and gefitinib by cationic lipid-PLGA nanoparticles improves EGFR-targeted therapy for glioma. J. Mol. Med. Berl. 97 (2019), 1575–1588. pmid: 31673738.
- [37] R. Dal Magro et al.: ApoE-modified solid lipid nanoparticles: A feasible strategy to cross the blood-brain barrier. J. Control. Release 249 (2017), 103–110. pmid: 28153761.
- [38] R. B. Brown: Public health lessons learned from biases in coronavirus mortality overestima- tion. Disaster Med. Public Health Prep. (2020), 1–24. pmid: 32782048.
- [39] V. Furer et al.: Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (2021). pmid: 33848321.
- [40] S. M. C. Tirado and K.-J. Yoon: Antibody-dependent enhancement of virus infection and disease. Viral immunology 16 (2003), 69–86. pmid: 12725690.
- [41] C.-T. Tseng et al.: Immunization with SARS coronavirus vaccines leads to pulmonary im- munopathology on challenge with the SARS virus. PLoS One 7 (2012), e35421. pmid: 22536382.
- [42] F. Negro: Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med. Wkly. 150 (2020), w20249. pmid: 32298458.
- [43] J. A. Tetro: Is COVID-19 receiving ADE from other coronaviruses? Microbes and infection 22 (2020), 72–73. pmid: 32092539.
-
[44] Y. Honda-Okubo et al.: Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J. Virol. 89 (2015), 2995–3007. pmid: 25520500.
Featured image is from Children’s Health Defense