9-Year-Old with No Pre-existing Conditions Died Two Weeks After Pfizer Shot, Latest VAERS Data Show
All Global Research articles can be read in 51 languages by activating the “Translate Website” drop down menu on the top banner of our home page (Desktop version).
To receive Global Research’s Daily Newsletter (selected articles), click here.
Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
***
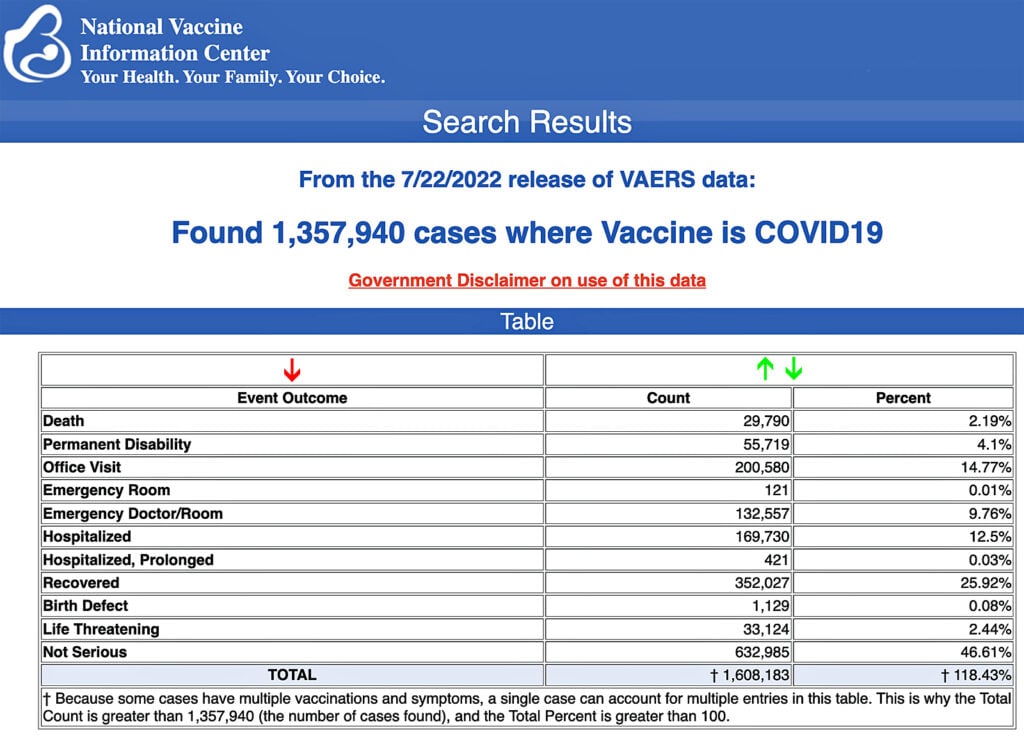
VAERS data released Friday by the Centers for Disease Control and Prevention show 1,357,940 reports of adverse events from all age groups following COVID-19 vaccines, including 29,790 deaths and 247,686 serious injuries between Dec. 14, 2020, and July 22, 2022.
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,357,940 reports of adverse events following COVID-19 vaccines were submitted between Dec. 14, 2020, and July 22, 2022, to the Vaccine Adverse Event Reporting System (VAERS). That’s an increase of 6,990 adverse events over the previous week.
VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 29,790 reports of deaths — an increase of 155 over the previous week — and 247,686 serious injuries, including deaths, during the same time period — up 1,010 compared with the previous week.
Of the 29,790 reported deaths, 19,236 cases are attributed to Pfizer’s COVID-19 vaccine, 7,917 cases to Moderna, 2,584 cases to Johnson & Johnson (J&J) and no cases yet reported for Novavax.
Excluding “foreign reports” to VAERS, 848,094 adverse events, including 13,805 deaths and 86,604 serious injuries, were reported in the U.S. between Dec. 14, 2020, and July 22, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 13,805 U.S. deaths reported as of July 22, 7% occurred within 24 hours of vaccination, 15% occurred within 48 hours of vaccination and 54% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 601 million COVID-19 vaccine doses had been administered as of July 20, including 355 million doses of Pfizer, 227 million doses of Moderna and 19 million doses of Johnson & Johnson (J&J).
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to July 22, 2022, for 6-month-olds to 5-year-olds show:
- 2,429 adverse events, including 81 cases rated as serious and 3 reported deaths.
- 4 reports of myocarditis and pericarditis (heart inflammation).
The CDC uses a narrowed case definition of “myocarditis,” which excludes cases of cardiac arrest, ischemic strokes and deaths due to heart problems that occur before one has the chance to go to the emergency department. - 15 reports of blood clotting disorders.
- 23 reports of seizures.
U.S. VAERS data from Dec. 14, 2020, to July 22, 2022, for 5- to 11-year-olds show:
- 12,232 adverse events, including 313 rated as serious and 9 reported deaths.
The most recent reported death involves a 9-year-old girl (VAERS I.D. 2377304) from California who died two weeks after receiving her first dose of Pfizer’s COVID-19 vaccine. The child experienced abdominal pain, sore throat and chest pain during the 2-3 days before she died, according to the VAERS report, which did not indicate any pre-existing conditions. - 24 reports of myocarditis and pericarditis.
- 47 reports of blood clotting disorders.
- 101 reports of seizures.
U.S. VAERS data from Dec. 14, 2020, to July 22, 2022, for 12- to 17-year-olds show:
- 32,835 adverse events, including 1,849 rated as serious and 44 reported deaths.
- 62 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 97% of cases attributed to Pfizer’s vaccine.
- 657 reports of myocarditis and pericarditis with 645 cases attributed to Pfizer’s vaccine.
- 165 reports of blood clotting disorders with all cases attributed to Pfizer.
- 20 cases of postural orthostatic tachycardia syndrome (POTS) with all cases attributed to Pfizer’s vaccine.
U.S. VAERS data from Dec. 14, 2020, to July 22, 2022, for all age groups combined, show:
- 20% of deaths were related to cardiac disorders.
- 54% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 73.
- As of July 22, 5,670 pregnant women reported adverse events related to COVID-19 vaccines, including 1,772 reports of miscarriage or premature birth.
- Of the 3,623 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 900 reports of Guillain-Barré syndrome, with 42% of cases attributed to Pfizer, 30% to Moderna and 27% to J&J.
- 2,293 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 1,743 reports of myocardial infarction.
- 14,254 reports of blood-clotting disorders in the U.S. Of those, 6,378 reports were attributed to Pfizer, 5,112 reports to Moderna and 2,718 reports to J&J.
- 4,273 cases of myocarditis and pericarditis with 2,619 cases attributed to Pfizer, 1,450 cases to Moderna and 188 cases to J&J.
- 14 cases of Creutzfeldt-Jakob disease with 8 cases attributed to Pfizer, 5 cases to Moderna and 1 case to J&J.
- 270 cases of POTS with 165 cases attributed to Pfizer, 87 cases to Moderna and 17 cases to J&J.
Children’s Health Defense (CHD) asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.
Woman develops rare acute kidney failure after first Pfizer dose
A woman developed a rare case of acute kidney renal failure — linked to antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) — a few days after receiving her first dose of Pfizer-BioNTech’s COVID-19 vaccine.
According to a case study published July 18 in Nephron, a previously healthy 47-year-old woman presented to a primary care clinic for bilateral flank pain, generalized weakness and bilateral lower extremity swelling that started three days after her first Pfizer shot.
AAV is a group of diseases characterized by the destruction and inflammation of small vessels. The condition occurs when neutrophils attack small and medium vessels of the body, which can affect several organs, such as the kidney, stomach, intestine and lungs.
This case adds to previous reports suggesting COVID-19 vaccines may, in rare instances, promote the development or worsening of autoimmune diseases, such as AAV, from their silent state, according to Patricia Inacio, Ph.D., who summarized the report for ANCA Vasculitis News.
“Rarely, autoimmune processes have been described post-vaccination. AAV is an example of an autoimmune disease that can be induced or flared up from a silent state by COVID-19 vaccines,” the authors concluded. “A high index of suspicion regarding the presence of an autoimmune renal process is needed whenever a recently COVID-19-vaccinated individual presents for acute kidney injury.”
43% of parents ‘definitely’ won’t vaccinate young kids for COVID
According to survey results released Tuesday, 43% of U.S. parents of children under 5 will “definitely not” give their child a COVID-19 vaccine amid concerns the vaccine poses a greater risk to kids than the virus.
The survey, published by the Kaiser Family Foundation, found that 27% of parents said they would “wait and see,” while another 13% said they would have their children vaccinated only if required to do so for school or childcare.
Even parents who were vaccinated against COVID-19 said they would not give permission for their youngest children to get vaccinated.
When asked why they will not vaccinate their eligible child under 5 “right away,” parents cited “concerns about the newness of the vaccine and not enough testing or research, concerns over side effects and worries over the overall safety of the vaccines.”
CDC used flawed data to justify authorizing COVID-19 vaccines for kids
A CDC official used data from a flawed preprint study that exaggerated the risk of death for children from COVID-19 in her presentations to CDC and FDA advisors who were responsible for recommending Pfizer and Moderna’s vaccines for infants and young children.
The study, first published May 25 on the medRxiv preprint server, was authored by a group of U.K. researchers. On June 28, the authors published a revised version of the study, after critics questioned some of their original findings.
“It’s really disturbing that data this poor made its way into the meetings to discuss childhood COVID and that it took me less than a few minutes to find a major flaw (and then I found many more as I looked deeper),” said Kelley K, who was the first to point out some of the study’s flaws on her website COVID-Georgia.com.
After learning of Kelley’s analysis, The Defender reviewed the original preprint, confirmed Kelley’s findings and uncovered additional flaws in the original preprint and also in the June 28 revised version.
CHD demands D.C. schools rescind COVID vaccine mandate
Robert F. Kennedy, Jr., chairman and chief legal counsel for CHD, in a letter to the superintendent of the District of Columbia school system threatened to sue the school district unless it rescinds its recently announced COVID-19 vaccine mandate for students ages 12 and up.
State Superintendent of Education Christina Grant announced on July 19 that student immunization requirements for the upcoming 2022-2023 school year will include the COVID-19 vaccine for all students who are of an age for which there is a vaccine fully approved by the FDA now that the FDA has fully approved the Pfizer-BioNTech vaccine for individuals 12 to 15 years old.
D.C. law requires students in all area schools, including private, parochial and independent schools, to be fully compliant with mandated vaccinations, unless they have an approved exemption. The law also requires schools to verify immunization certification for all students.
The requirement was detailed in a law the D.C. Council approved last year and is the first legislation of its kind in the region.
Although courts have upheld many childhood vaccination requirements for licensed and approved vaccines, no court has ever upheld a mandate for schoolchildren for an Emergency Use Authorization vaccine, according to Kennedy.
*
Note to readers: Please click the share buttons above or below. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
Megan Redshaw is a staff attorney for Children’s Health Defense and a reporter for The Defender.
Featured image is from CHD

